Chemistry A1 - Relating properties to uses and production of substances
1/64
Earn XP
Description and Tags
Textbook, not completed
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
65 Terms
Common name for aluminium oxide?
Alumina
One property of alumina?
It is amphoteric
What does amphoteric mean?
A substance that can act as both an acid or a base, depending on the conditions
What does base mean?
A compound that reacts with an acid to form a salt and water
What does acid mean?
A compound containing hydrogen that dissociates in water to form hydrogen ions
What does aluminium oxide react with hot hydrochloric acid give?
Aluminium chloride and water
What is the aluminium chloride?
Soluble
Equation for the above statement?
Al2O3 + 6HCL → 2AlCl3 + 3H2O
Aluminium oxide is chemically inert except under certain conditions. Give an example
When a hot acid or base is present.
What does that mean?
It means that it has a lot of uses which includes filler, paint, sunscreens, and glass.
What does the amphoteric nature of alumina mean?
It means that it can be used as a medium for chromatography as a basic, acidic, or neutral medium
What is alumina also?
An effective desiccant
What range can it be used at?
A range of basic and acidic pHs
At what range is it stable at?
Stable over pH range 2-13
For what does it make suitable for?
Environmental clean-up and separation applications
What are group 1 and 2 metal oxides?
Basic
What happens when they dissolve in water?
They will react with the water to form metal hydroxides
What does that form?
An alkali solution
Select the 1 correct example for an alkali solution
Na2O + H2O - > 2NaOH
Select the other correct example for an alkali solution
CaO + H2O - > Ca(OH)2
What is an alkali?
A base that dissolves in water to form hydrogen ions
What is the oxide ion?
A very strong basic anion
This is due to?
It very small size and high (-2) charge
What does the oxide ion in the metal oxide react with water to produce?
Hydrogen ions
Why?
Because a hydroxide ion is the strongest base that can persist in water
What is another name for calcium oxide?
Quicklime
What does calcium oxide react with water to form?
Calcium hydroxide
Another name for calcium hydroxide?
Lime
What can calcium hydroxide be used for?
Used by farmers to raise the pH of acidic soil
What can magnesium oxide be used for?
As a desiccant when preserving books in a library
Is magnesium oxide a good desiccant?
No
Why is it used for?
Used because due to its basic nature, it will neutralise the acidic sulfur oxides produced, so it helps preserve the book.
How is sodium hydroxide produced?
Sodium oxide reacts with water
What is it used for?
Used in some processes to make plastics and soaps
Another use?
Used in food processing in many ways
Examples?
To peel fruits and to process cocoa and chocolate
Where is it found?
In drain cleaner or in oven cleaner
What is magnesium hydroxide also known as?
Milk of magnesia
What is it used for?
Treating acid indigestion
How?
Raises the stomach pH
Due to?
Its basic nature
What is calcium hydroxide used for?
In the treatment of acidic effluent
Factories that produce or used sulfuric acid also produce?
Acidic liquid effluent
Does it need to be treated before released to the environment
Yes, especially if it has high acid content
Impurities are precipitated and removed. The acidic liquid can be neutralised using?
Calcium hydroxide
What is the reaction for this?
H2SO4 + Ca(OH)2 → CaSO4 + 2H2O
What is the calcium hydroxide?
Base
What is formed?
Neutral calcium sulfate
What is calcium sulfate?
A salt
Why is calcium hydroxide used?
Cheap and simple to use
What does salt mean?
A compound formed by an acid-base reaction where the hydrogen in the acid has been replaced by a metal (or other positive) ion
What is electrolysis?
The decomposition of a compound using electricity
What does electrolysis break down?
Compounds into simple substances
Where is it used for?
In some industrial processes
Ionic substance are…
Decomposed by an electrical charge being passed through them during electrolysis
How must the ions be to be able to do this?
Must be free to move for this work
How must the compound be?
Either molten or in solution
What is molten?
Reduced to liquid form by heating
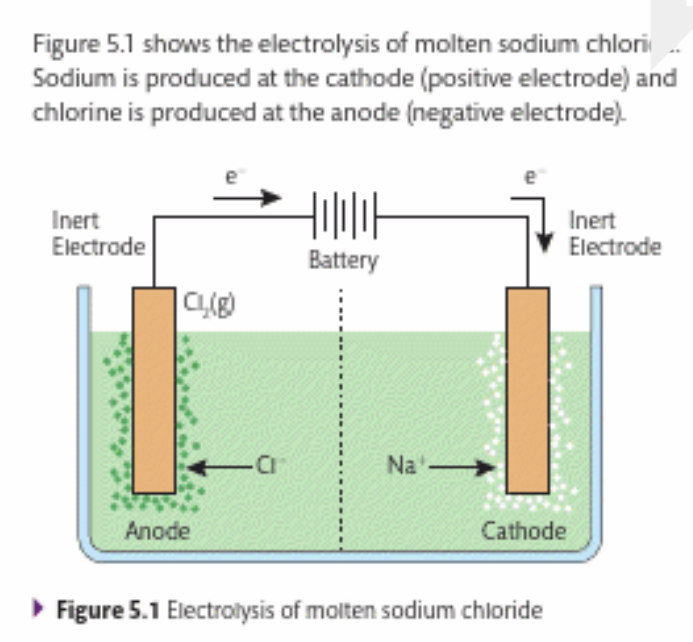
What is produced by this reaction?
Only sodium and chloride
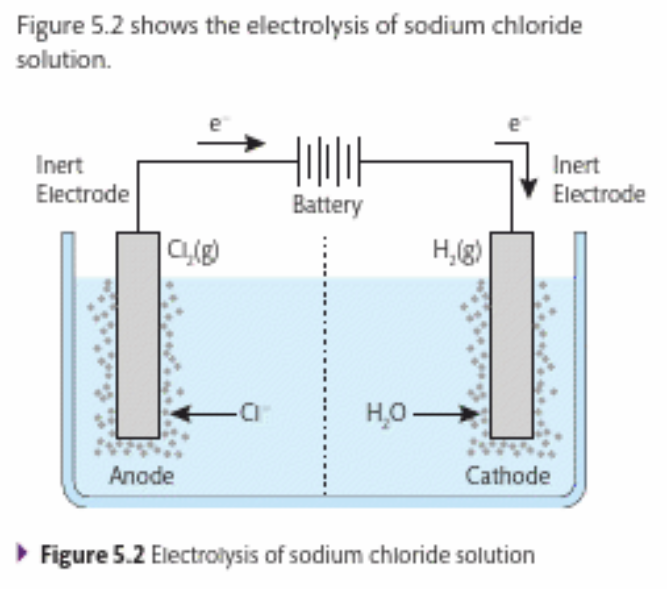
What is present when sodium chloride is dissolved in water?
Hydrogen and hydroxide ions, also sodium and chloride ions
What is produced at the anode?
Chlorine
What is produced at the cathode?
Hydrogen gas
Why is hydrogen gas produced at the cathode instead of sodium?
Because it is only possible for one type of ion to discharge at each cathode
How do you know which ion is selected to discharge at the electrode?
Depends on a number of factors like the position of the ion in the electrochemical series affects its ease of discharge at an electrode.
When metals react, they lose electrons to become positive ions. If a metal is placed in water, then the metal atoms will tend to lose electrons to the water and become positive. This in turn attracts the negative electrons back to the metal. Example of an equilibrium process.