Bonding and physical properties
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
49 Terms
What are state changes?
Physical changes that can be reversed
Do state changes change the chemical properties or chemical makeup of the substances involved?
No
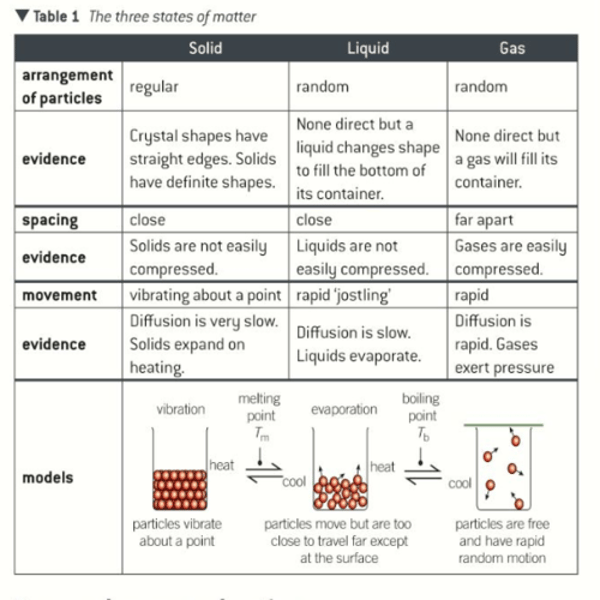
States of matter table
Heating a solid:
What happens when you first heat a solid? What does this cause in the solid?
As energy is supplied to the particles they vibrate about a fixed position.
This causes a slight increase in the average distance between the particles and so the solid expands
Solid to liquid:
What is melting also called?
Fusion
What happens to a solid when energy is supplied for it to melt?
The forces which act between the particles that holds them in a solid state are weakened
What is the energy needed to melt a solid called?
The enthalpy change of melting/fusion
Why does the temperature not change when a solid is melting?
Because the heat energy provided is absorbed as the forces between the particles are weakened
What is the difference between enthalpy and temperature?
Enthalpy is the heat energy change measured under constant pressure.
Temperature depends on the average kinetic energy of the particles - related to the speed
What happens when you heat a liquid?
More energy is supplied to the particles which makes them move more quickly - more kinetic energy.
What do liquids do when heated?
They expand
Liquid to gas:
What is boiling also called?
Vaporisation
What is required to turn a liquid into a gas?
Enough energy needs to be supplied to break all the intermolecular forces between the particles
What is the energy needed to turn a liquid into a gas called?
Enthalpy change of vaporisation
Is there a temperature change during boiling?
No
What happens when you heat a gas?
The particles gain kinetic energy and move faster. They get much further apart and so gases expand a lot when heated
What are crystals and what are their arrangements?
Solids whose particles are arranged in a lattice structure held together by forces of attraction
What are the possible forces of attraction?
Strong bonds - such as covalent, ionic or metallic - or weak intermolecular forces: van der Waals, dipole-dipole or hydrgrogen bonds
What does the strength of the forces between the particles in the crystal affect?
The physical properties of the crystal
What are the four basic crystal types?
Ionic, metallic, molecular and macromolecular
What is the attraction within ionic compounds?
Strong electrostatic compounds between oppositely charged ions
Structure of sodium chloride
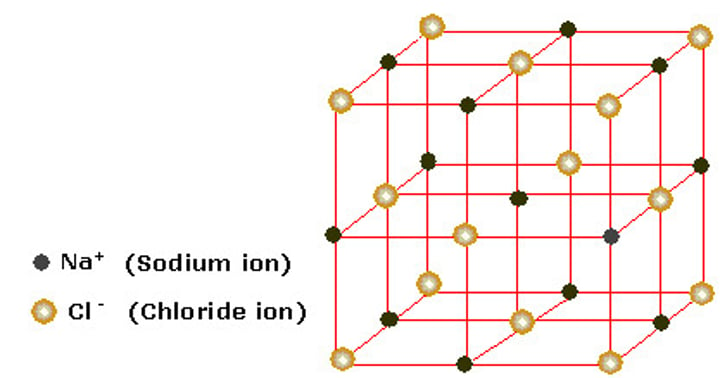
Why do ionic compounds have high melting points?
The strong electrostatic attractions between oppositely charged ions require a lot of energy to break
How do metals exist?
As a lattice of positive ions embedded in a delocalised sea of electrons.
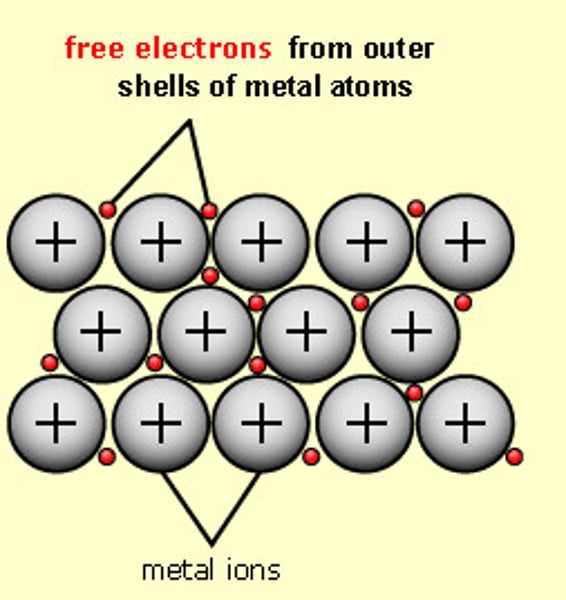
Metallic bonding of magnesium
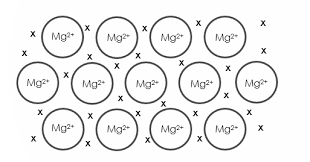
Why do metals have high melting points?
There is a strong force of attraction between the negatively charged delocalised 'sea' of electrons and the positive metal cations
What do simple molecular crystals consist of?
Molecules held in a regular array by intermolecular forces
What type of bonds hold the atoms together in molecular crystals?
Covalent bonds
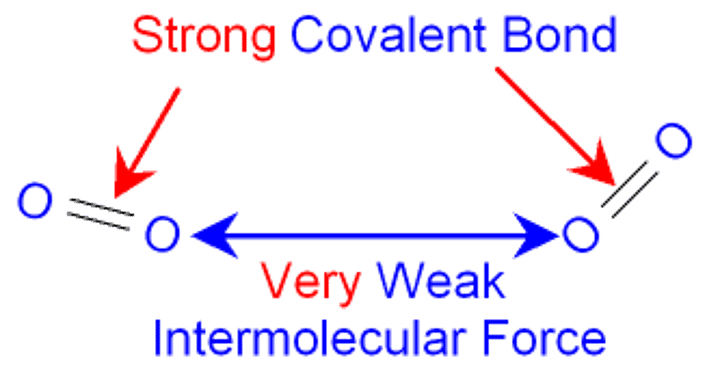
Do covalent bonds act between the molecules?
No - intermolecular forces do
Why do simple molecular crystals have low melting temperatures and low enthalpies of melting?
Because intermolecular forces are very weak
Structure of iodine
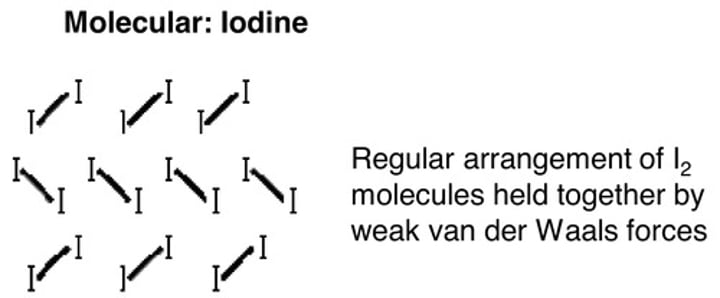
Why is iodine a solid at room temperature?
Iodine molecules have a large number of electrons so the van der Waals forces are strong enough to hold the molecules together
What are the properties of iodine? (3)
crystals are soft and easy to break.
low melting temperature and sublimes readily to form gaseous iodine molecules.
does not conduct electricity as there are no charged particles to carry charge.
How do macromolecular crystals differ from simple molecular?
Macromolecules are simple molecules, but they contain large numbers of atoms which are linked by covalent bonds in a regular 3D lattice
What are examples of macromolecular crystals?
Diamond and graphite
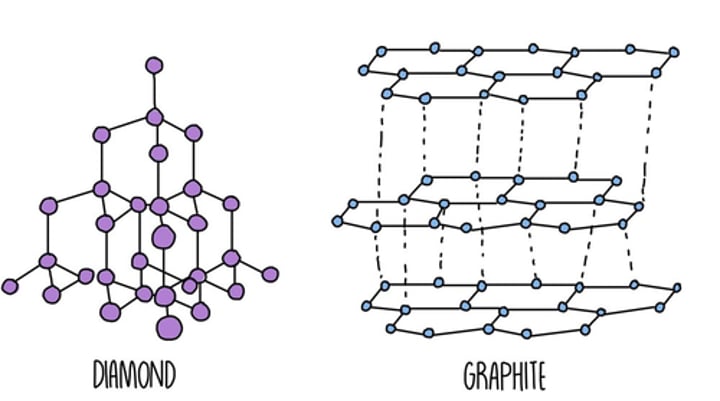
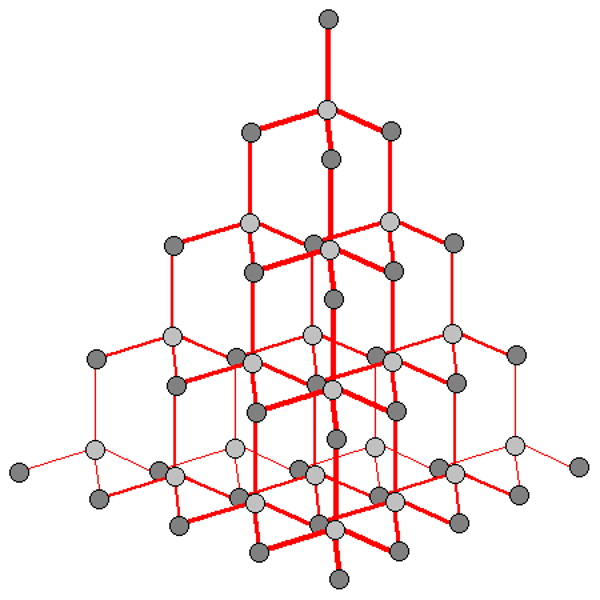
What is the structure of diamond?
A giant covalent structure in which each carbon atom is joined to four other carbon atoms by strong covalent bonds
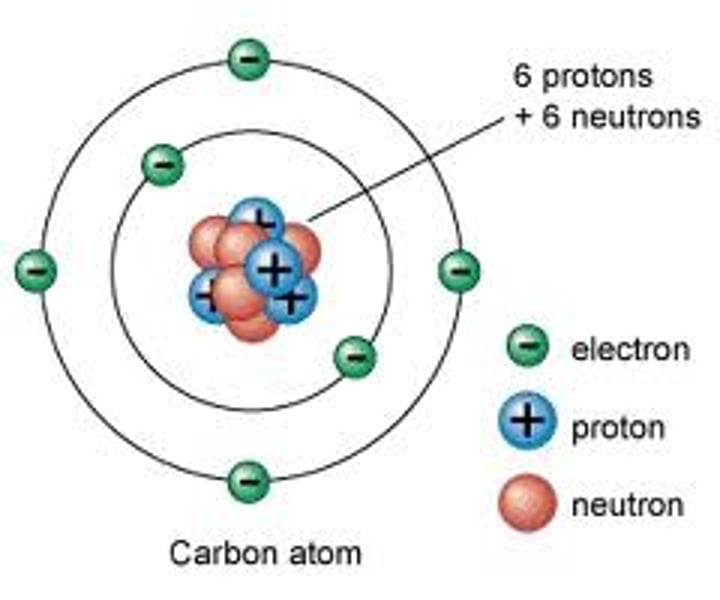
Electron configuration of carbon
1s² 2s² 2p²
How many covalent bonds does each carbon atom form in diamond?
Four
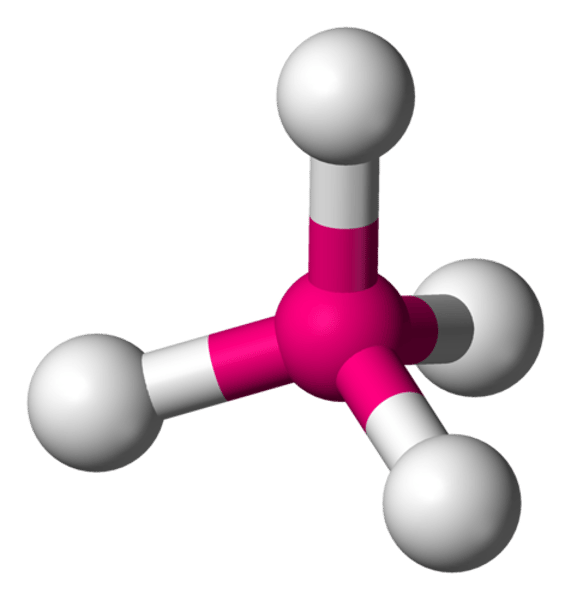
What shape does the four covalent bonds form, as predicted by the bond electron pair repulsion theory?
Tetrahedral
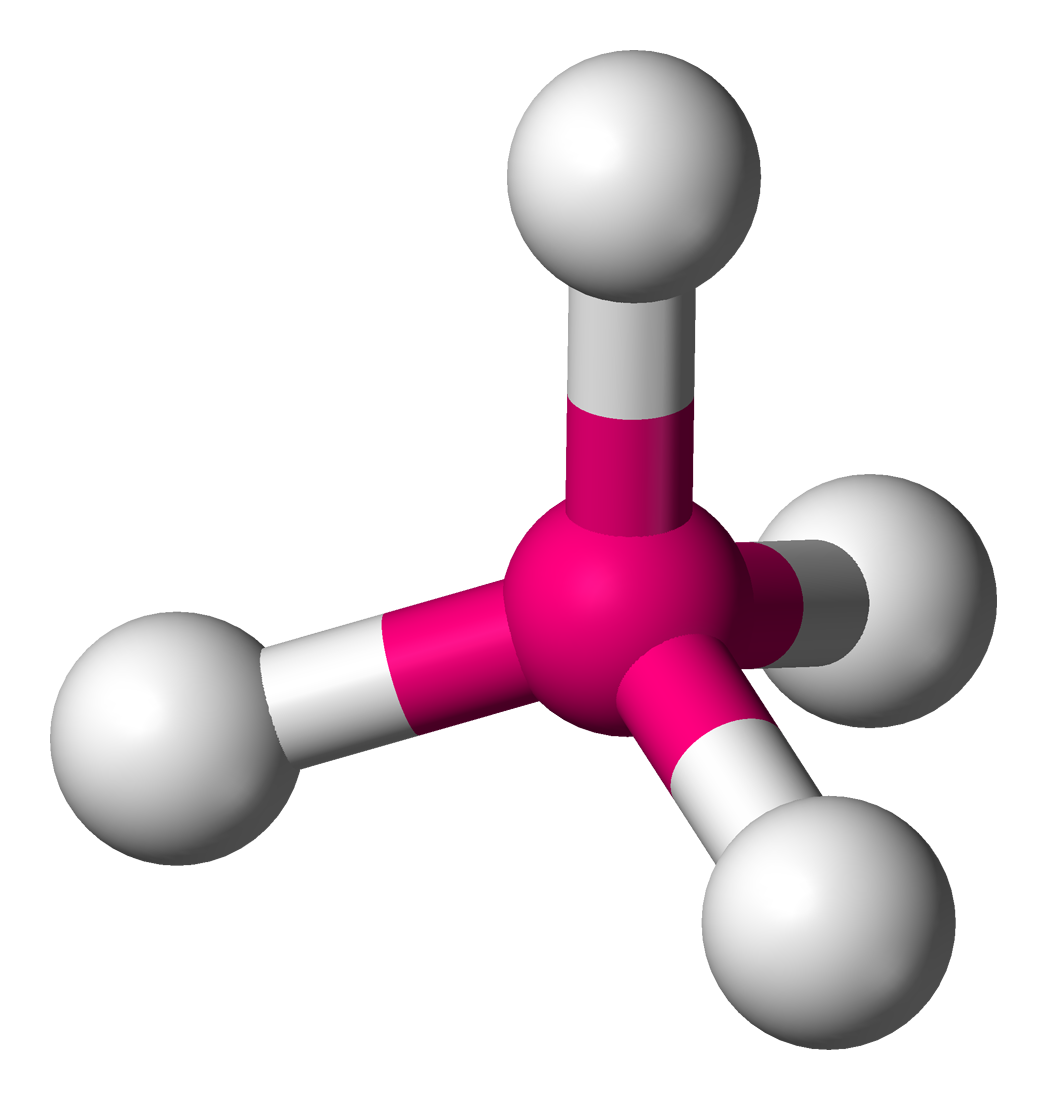
What are the properties of diamond?
high melting point,
very hard,
does not conduct electricity
What two sorts of bonding does graphite have?
Covalent bonding and van der Waals forces
How many covalent bonds does each carbon atom form in graphite?
Three
What shape does the three covalent bonds form, as predicted by the bond electron pair repulsion theory?
Trigonal planar
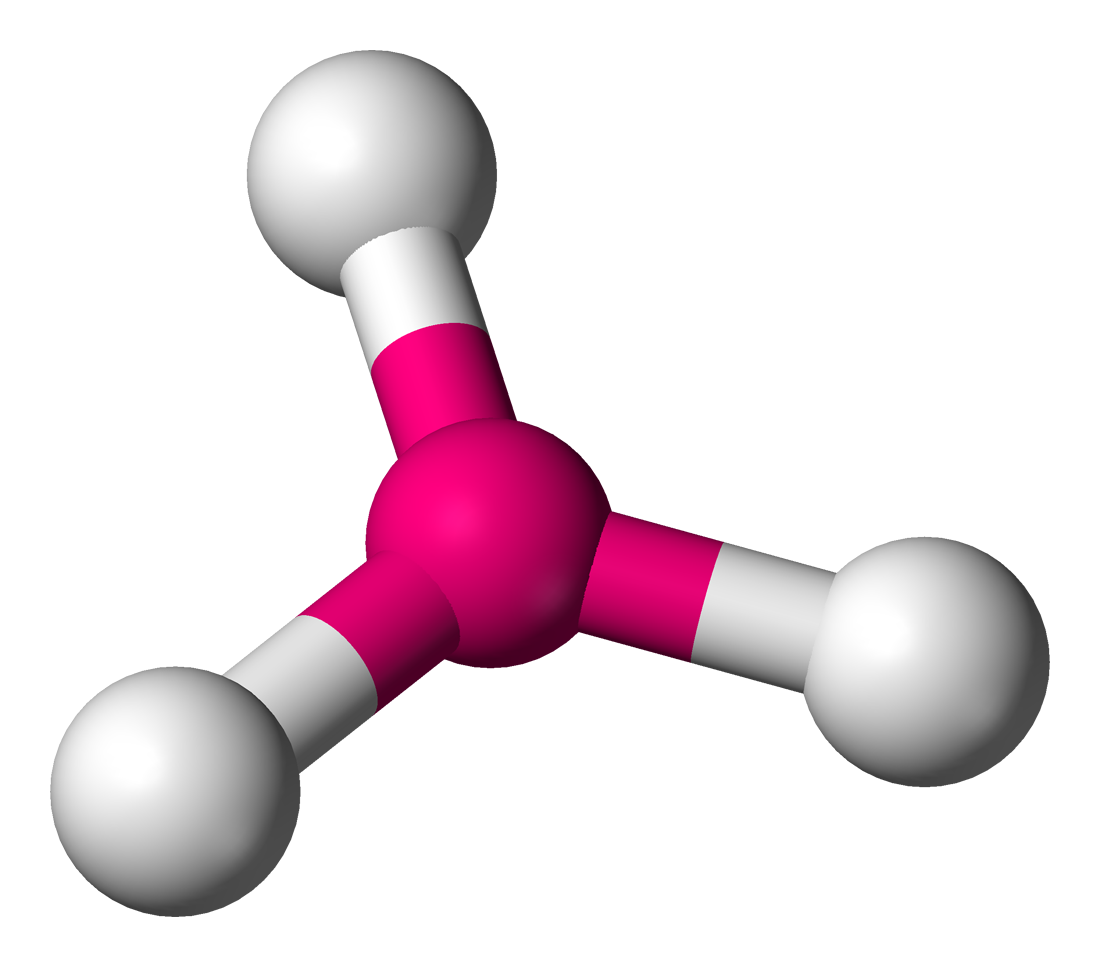
How does the structure of graphite differ from that of diamond?
Each carbon atom is left with a ‘spare’ electron in the p-orbital that is not apart of the covalent bond
What is the structure of graphite?
Giant covalent structure in which each carbon atom forms three covalent bonds with other carbon atoms. The carbon atoms form layers of hexagonal rings and the layers are held together by weak Van der Waals forces
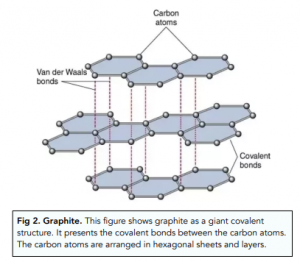
What can the ‘spare’ electrons from each carbon do?
They are delocalised and can move within the layer. These delocalised electrons are what allows graphite to conduct electricity
How does graphite conduct electricity?
They only conduct electricity across the hexagonal planes, not at right angles to them
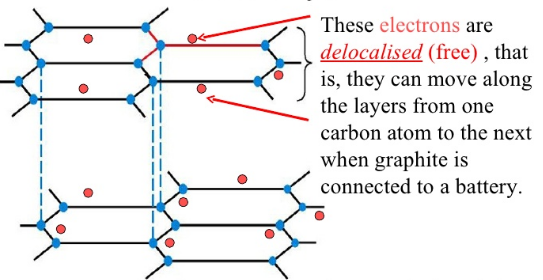
What do the weak intermolecular forces between graphite allow the layers of graphene to do?
They can slide across one another, which makes graphite soft
What are the properties of graphite?
Soft material
Very high melting point - graphite even breaks down before it melts
Conducts electricity along the hexagonal planes