Key Concepts in Chemistry
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
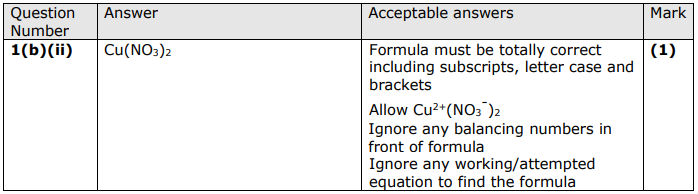
Write the formula for copper nitrate
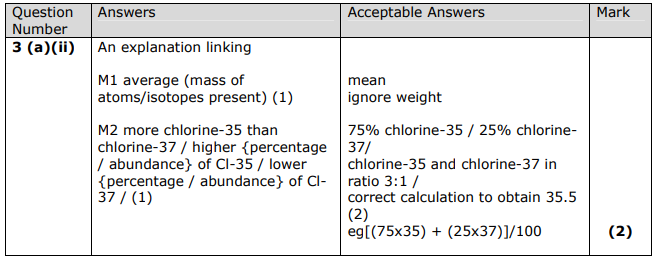
A normal sample of chlorine contains only chlorine-35 and chlorine-37 atoms.
Explain why the relative atomic mass of chlorine is 35.5
Relative atomic mass measures the average mass of isotopes present
There is a higher abundance of Cl-35 than Cl-37
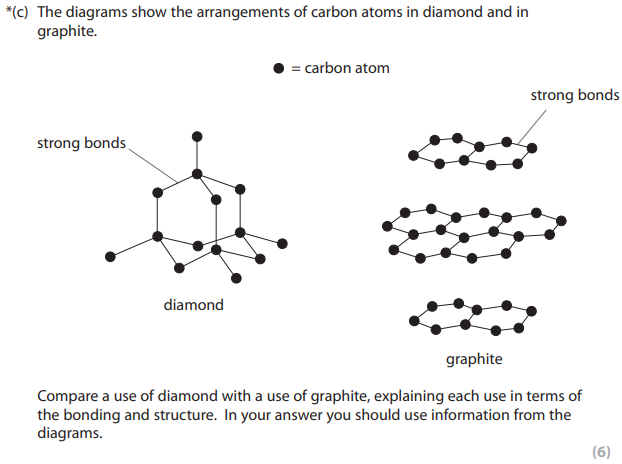
Compare a use of diamond with a use of graphite, explaining each use in terms of the bonding and structure. In your answer you should use information from the diagrams.

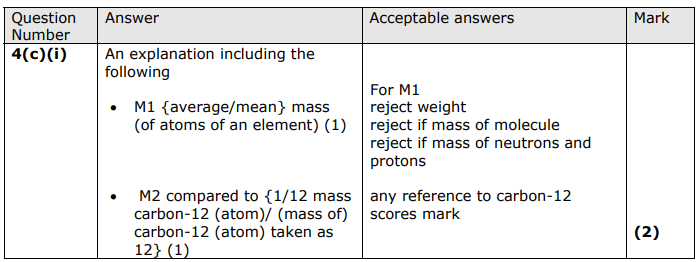
Explain what is meant by the term relative atomic mass.
The average mass of an elements atoms
Compared to 1/12 of the mass of a C-12 atom
Mendeleev was a Russian chemist who produced the first version of the periodic table.
Give one similarity and one difference between his version of the periodic table and the periodic table shown on page 2.
Both periodic tables put elements with similar properties in the same group
However Mendeleev’s table had gaps