Using materials
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Gold and other very unreactive metals do not oxidise in air at all.
What is corrosion?
Happens when a metal continues to oxidise. The metal becomes weaker over time and eventually all of it may become metal oxide.
What is rusting?
Rusting is an example of corrosion. It occurs when iron or steel reacts with oxygen and water:
iron + oxygen + water → hydrated iron(III) oxide
Hydrated iron(III) oxide is the orange-brown substance seen on the surface of rusty objects.
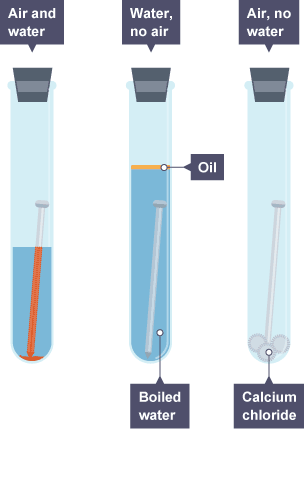
A rusting experiment
The experiment in the diagram shows that both oxygen and water are needed for rusting to happen.
The nail only rusts in the left-hand test tube. It does not rust:
in the middle test tube, where there was water but no oxygen (because there was no air in the water)
in the right-hand test tube, where there was oxygen (air) but no water
Explain whether iron is oxidised or reduced when it forms rust.
Iron is oxidised because it gains oxygen during rusting.
Preventing corrosion
Removing substances that cause rusting
Rusting can be prevented by keeping oxygen or water away from the iron or steel: 2
oxygen can be excluded by storing the metal in an atmosphere of unreactive nitrogen or argon
A desiccant is a substance that absorbs water vapour, so it keeps the metal dry.
Physical barriers to oxygen and water: 3
painting
oiling and greasing
coating with plastic
Explain why a bike chain is protected from rusting by oiling it, rather than by painting it.
The oil lubricates the chain, helping it to move smoothly. Paint just flakes off when the bike is ridden, exposing the steel chain to air and water again.
Uses of metals
Most metals in everyday use are alloys.
What is an alloy?
An alloy is a mixture of two or more elemetns, where at least one element is a metal.
Copper alloys
Bronze was the first alloy to be made by humans, around 6000 years ago.
What is bronze made from?
What is it used to make?
copper and tin
statues, bells and coins
Gold
Gold is a very soft and malleable metal. It is also very unreactive, so it resists corrosion and stays shiny. The visors on space helmets are coated with a layer of gold. This is thin enough for the astronaut to see through but thick enough to reflect sunlight
to be continued… (bbc bitesize)