T2 Chemistry of Life
1/22
Earn XP
Description and Tags
Learning Outcome: Chemical Context of Life - Describe the molecular characteristics that influence biological activity and apply these characteristics to address biological questions - Describe various chemical bonds, infer the biological importance of each type of bond and apply knowledge of these bonds to predict how molecules and compounds might behave. - Understand the structure of some important biological molecules Water is life - Explain how covalent bonds and hydrogen bonds influence the behaviour of water molecules - Describe the following properties of water molecules: adhesion, cohesion, surface tension and specific heat Biological Molecules - Identify important biological molecules and their roles in living organisms: carbohydrates, proteins, nucleic acids and fats
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
is water polar? or non polar?
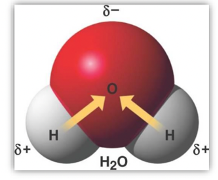
Water is a polar molecule, as it contains polar bonds (type of covalent bond where electrons are shared unequally between two atoms)
The negative side of one water molecule is attracted to the positive side of another.
This polarity allows water to form hydrogen bonds, which are crucial for many of its unique properties, including high surface tension and solvent capabilities.
What are the biological significances of bonds?
Weak chemical bonds reinforce shapes of large molecules and help molecules adhere to each other
Weaker ionic and hydrogen bonds used in molecules that require minor changes to activate a reaction
Strong covalent bonds used in stable molecules
A molecule’s biological function is related to its 3D shape
The shape of a molecule is determined by the positions of the electron orbitals that are shared by the atoms involved in the bonds
Explain how covalent bonds and hydrogen bonds influence the behaviour of water molecules
Covalent bonds within water molecules (O-H) lead to a polar molecule (with a slightly negative oxygen and slightly positive hydrogen)
hydrogen bonds between water molecules (attraction of positive hydrogen to negative oxygen of another molecule) cause water's unique properties like high boiling point and cohesion
What are the 4 properties of water?
Versatile solvent (via hydrogen bonding)
Cohesion (via hydrogen bonding)
High specific heat
Expansion upon freezing (ice floats)
what does it mean by water is a versatile solvent?
Water can dissolve many substances due to its polar nature, allowing it to interact with various ions and molecules.
The negative end of a water molecule is attracted to cations
The positive end of the water molecule is attracted to anions
Water molecules form a sphere around the ions in an ionic compound and it dissolves
what does it mean by cohesion (via hydrogen bonding)
Water clings to itself and other structures: Cohesion
Hydrogen bonding ensures that water holds together (cohesion).
Water is more structured than other liquids
Hydrogen bonding allows water to cling to biological material like the plant cell wall (adhesion)
whats the difference between cohesion and adhesion, in relation to water in a tree’s vascular system
Cohesion: attraction between molecules of the same substance
Water clings to itself and other structures due to hydrogen bonds, that hold the substance together
- Helps to counter the effects of gravity in plants
- As water evaporates through the leaf, cohesion in hydrogen bonds helps pull water molecules up and holds them together as a column of water in the cells
Adhesion: attraction between molecules of different substances
the clinging of a substance to another
- Adhesion of water molecules to cell walls assists them to get pulled up and counter the downward pull of gravity
what does high specific heat mean in relation to water?
Water resists changes in temperature
It takes a lot of heat/energy to increase the temperature of water
Cooling water causes a lot of heat/energy to be released.
Water has a high specific heat compared to other substances
what is specific heat?
- Specific heat: the amount of heat that must be absorbed to result of a change in temperature
- Specific heat of water is 1 calorie per gram per degree Celsius
- Water resists changes in temperature, thus high specific heat.
what is Expansion upon freezing (ice floats)
Water is less dense as a solid
Instead of contracting and densifying, it expands ( due to hydrogen bonding)
Allows ice to float
As water cools the molecules slow down
Eventually they start to from a stable structure
A crystalline structure with “holes”
This makes the frozen water (ice) less dense than liquid water (which does not have holes)
If ice sank, eventually all ponds and lakes would become completely solid, making life impossible
What are the 4 classes of large biological molecules?
carbohydrates
proteins
nucleic acids
lipids – various shapes and lengths
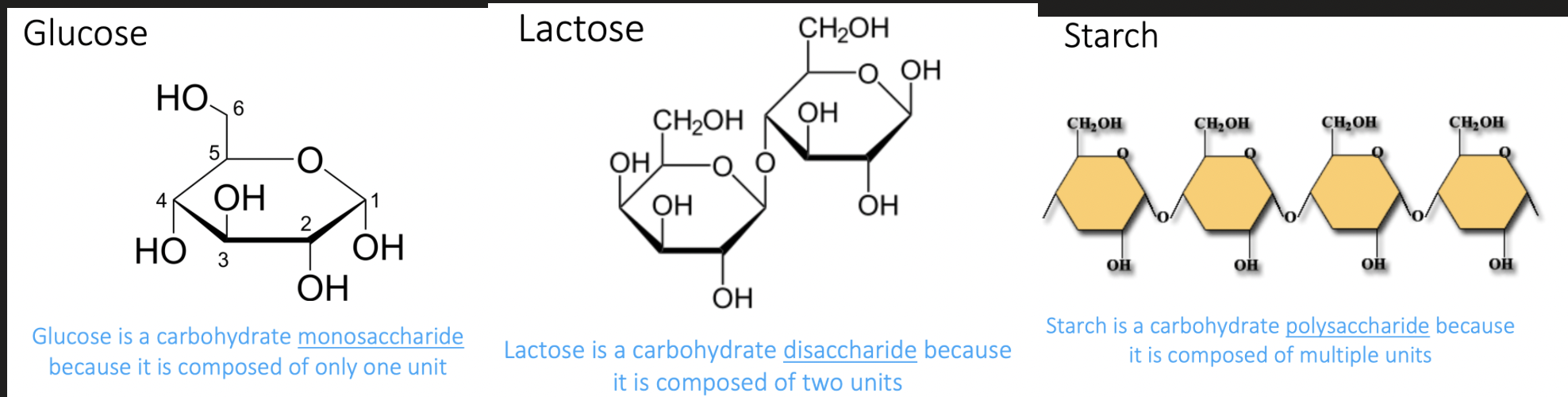
what are carbohydrates?
Serve as fuel and building material
Monosaccharides (one monomer, e.g. glucose), Disaccharides (two monomers, e.g. lactose), Polysaccharides (many monomers, e.g. starch) - cellulose (plants) - starch (plants) - glycogen (animals) - chitin (animals and fungi)
what are proteins?
Form enzymes, structural building blocks, hormones, transport, defence proteins, etc.
Built from a suite of 20 amino acids [amino acids all have a carboxyl and an amino group]
what are the 4 different ways to illustrate protein structures?
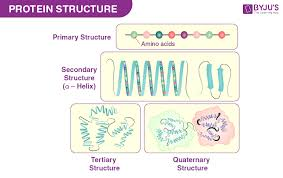
Structure | Bonds holding the structure together | Short description of the structure | Number of polypeptide chain involved |
Primary | Peptide bonds | Linear chain of amino acids | 1 |
Secondary | Hydrogen bonds Disulfide bridges | Regions stabilised by hydrogen bonds between atoms of polypeptide backbone | 1 or more |
Tertiary | Hydrogen bonds Van der Waals forces Disulfide bridges | Three dimensional shape stabilised by interactions between side chains | 1 |
Quaternary | Hydrogen bonds Van der Waals forces | Association of two or more polypeptides | Two or more identical |
what happens to a protein during denaturing?
- Disruption of hydrogen, van der Waals and disulphide bridges
- Protein loses its shape and ability to function
Which of the four levels of protein structure is maintained after denaturing? Explain your answer.
Primary
all other structures have weak bonds that are broken easily (hydrogen and van der Waals)
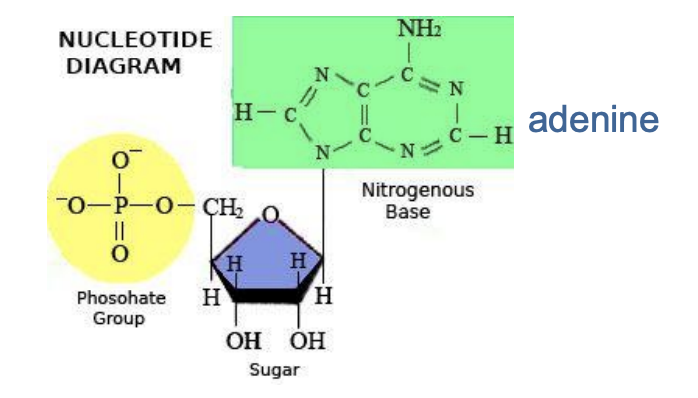
what are nucleic acids?
Store, transmit and express heredity in DNA and RNA
Five (5) bases: A, T, C, G, U (adenine, thymine, cytosine, guanine, uracil)
Each base contains a phosphate group and a nitrogenous base:
what are lipids?
Serve as fuel or building blocks
Various shapes and length
Fats e.g. triacylglycerol (1 glycerol + 3 fatty acids, e.g. fat)
Phospholipids (1 phosphate group + 2 fatty acids, e.g. lipid bilayer of membranes): found in cell membranes
Cholesterol
Steroids (4 cyclic rings, e.g. cholesterol, estradiol)
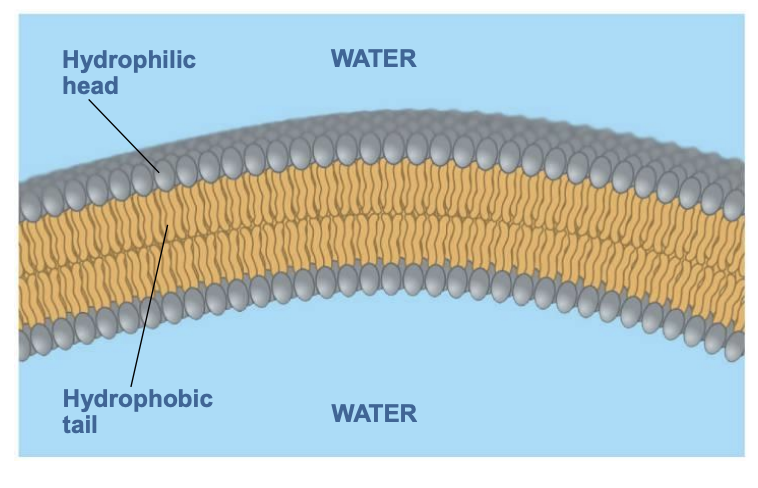
what are covalent bonds?
Sharing of a pair of valence electrons by two atoms.
Achieves completed valence shells
strong
what are polar bonds?
a type of covalent bond where electrons are shared unequally between two atoms
Polar covalent bonds can result in a polar molecule – one with a positive and negative side.
what are hydrogen bonds?
Hydrogen atoms in a polar molecule are attracted to electronegative atoms in another molecule with a hydrogen bond
Hydrogen bonds are WEAK
what are ionic bonds?
strong. Between oppositely charged ions. Can form between any two oppositely charged ions
what are Van Der Waals forces?
weak.
Attraction between molecules caused by localised charge fluctuations when the e- moves around the nucleus