orgo ch 1
1/56
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
57 Terms
alkanes
cn hnplus2
1 carbon
meth
2 carbon
eth
3 carbon
prop
4 carbon
but
5 c
pent
6 carbon
hex
7 carbon
hept
8 carbon
oct
9 carbon
non
10 carbon
dec
primary carbon
carbon attached to anther carbon
secondary carbon
carbon attached to 2 other carbons
tertiary carbon
carbon attached to 3 other carbon
quaternary
carbon attached to 4 other carbon
constitutional isomers
same molecular formula but different connectivity of bonds, same degree of unsaturation
stereoisomers
same molecular formula, same connectivity of bonds but different orientation of groups
alkyl group
substituent group derived from alkane by removing h atom
cycloalkanes
cn h2n
alkenes
have double bond, adiene, atriene
alkyne
have triple bond
iupac rule
lower number to suffix function group, then multiple bonds, then substituents
carboxylic acid
cooh, oic acid
sulfonic acid
so3h, sulfonic acid
ester
coo, oate
acid chloride
cocl, oyl chloride
amide
conh2, amide
nitrile
cn, nitrile
aldehyde
cho, al
ketone
co, one
alcohol
oh, ol
thiol
sh, thiol
amine
nh2, amine
positional isomers
constitutional isomers, same mf, different properties due to different position of fg
functional isomers
constitutional isomers, same mf, different properties due to different functional groups
degree of unsaturation
total number of multiple bonds and cyclic rings
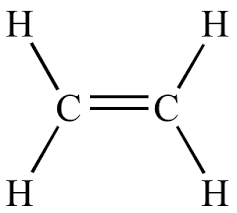
vinyl
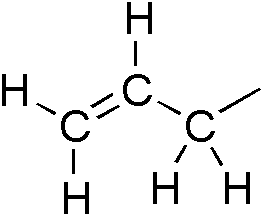
allyl
nh2 prefix
amino
br prefix
bromo
cl prefix
chloro
cn prefix
cyano
f prefix
fluoro
cho prefix
formyl
oh prefix
hydroxy
i prefix
iodo
no2 prefix
nitro
sh prefix
mercapto
o prefix
oxo
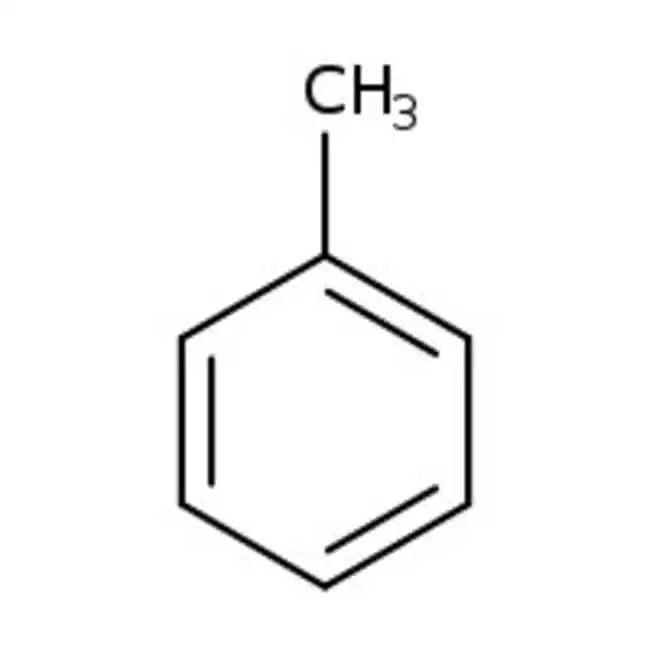
toluene
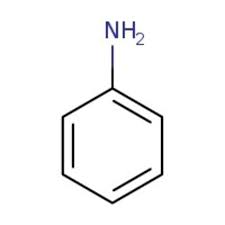
aniline
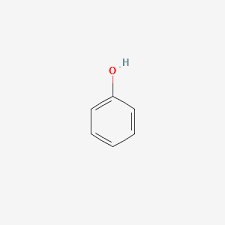
phenol
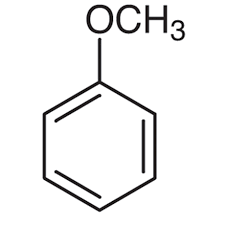
anisole
isopropyl
rchch3ch3
sec butyl
rchch3ch2ch3
tert butyl
rcch3ch3ch3
isobutyl
rch2chch3ch3