Unit 4- Aldehydes & Ketones
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
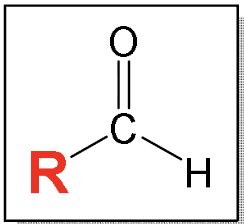
Aldehyde: carbonyl group (c double bond O) between a hydrocarbon-type carbon (only C and H)
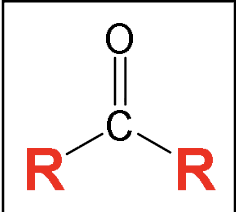
Ketone: carbonyl group (C double bond O) between hydrocarbon-type carbons (C and H)
What is the boiling point for an aldehyde?
49.0 C
What is the boiling point for a ketone?
56.0 C
What are the intermolecular forces of aldehydes and ketones?
Strong dipole-dipole forces
IUPAC aldehyde nomenclature
find longest continuous carbon chain containing carbonyl carbon
remove “-e” from corresponding alkane name and replace it with “-al”
number parent chain giving “1” designation to carbonyl carbon
name and number substituents and continue accordingly
IUPAC ketone nomenclature
find longest continuous carbon chain containing carbonyl carbon
remove “-e” from corresponding alkane name and replace it with “-one”
number parent chain giving “1” designation to carbonyl carbon
prefix parent name with number of ketone carbonyl
name and number substituents and continue accordingly
Ketone common nomenclature
3 words d
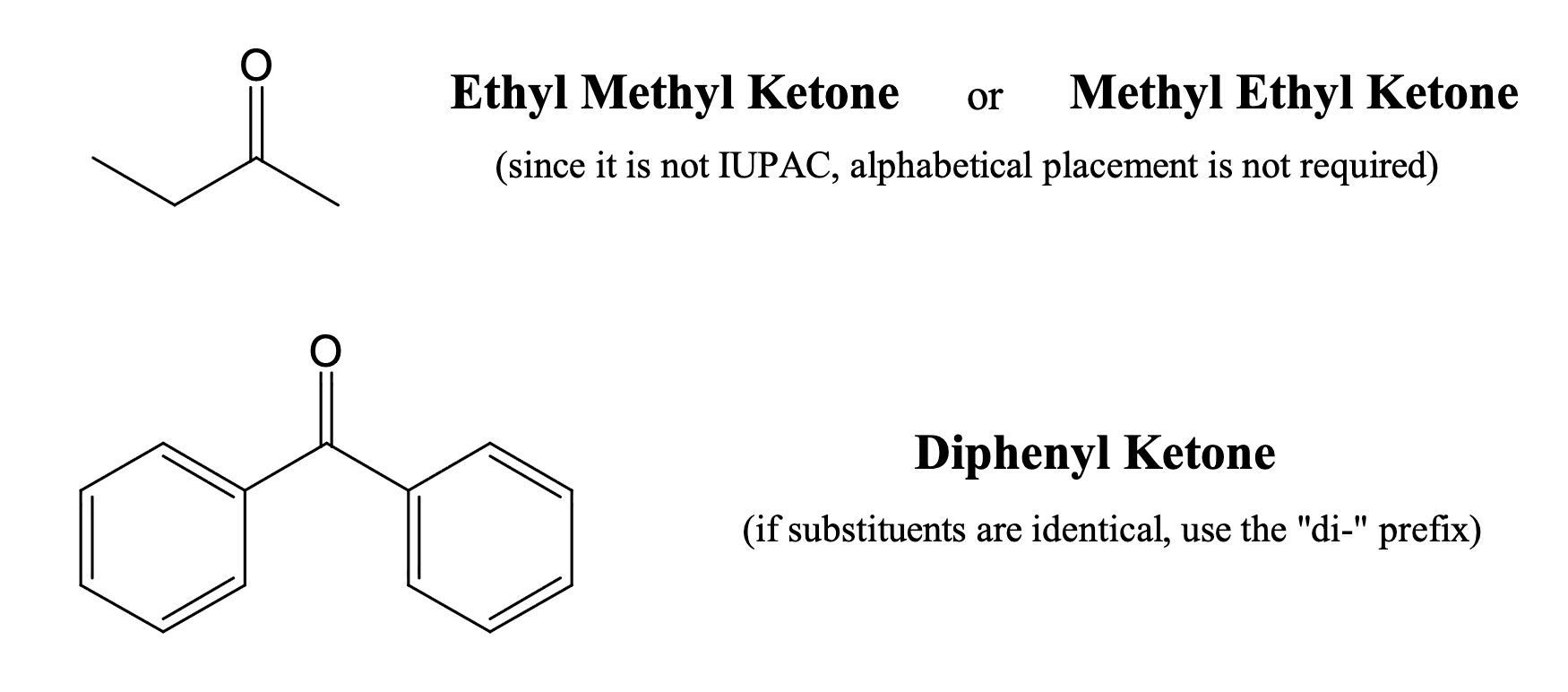
describing carbon architecture around the ketone carbonyl
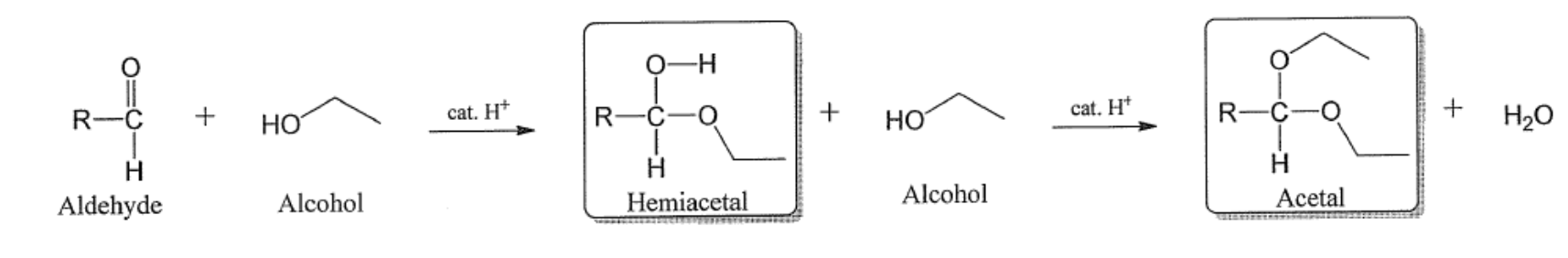
Condensation reaction of aldehyde
Condensation reaction for ketone
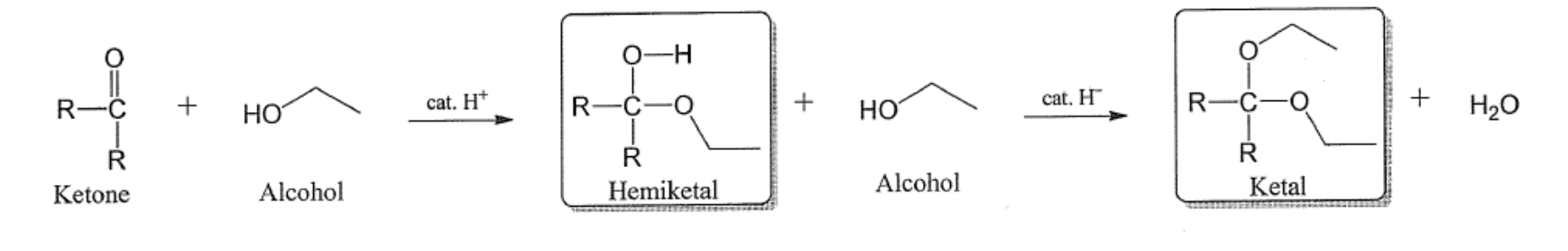
Ketal vs Acetal?
Is there hydroxyl (-OH) directly attatched to central carbon?
—> yes: “hemi” in name
—> no: “hemi” not in name
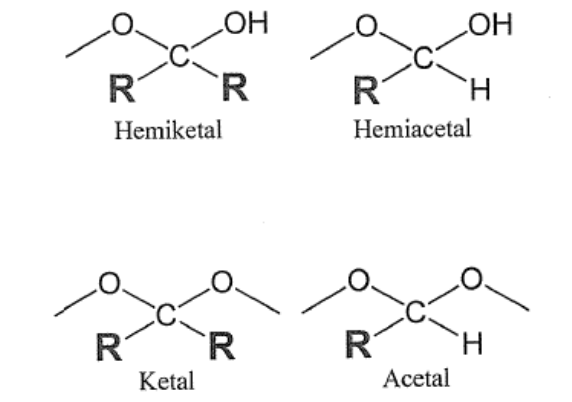
Is there a hydrogen (-H) directly attached to central carbon?
—> yes: word contains “acetal”
—> no: word contains “ketal”