pain pathways
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
define pain
unpleasant sensory and emotional experience associated with actual or potential tissue damage or described in terms of such damage
what is nociception
mechanism by which noxious peripheral stimuli are transmitted to CNS
how does nociception differ from pain
nociception is signal whilst pain is perception and emotional interpretation
what is a noxious receptor (aka nociceptor)
nerve ending that detects damaging stimuli
name the different types of pain
nociceptive/acute pain
chronic
neuropathic
central neuropathic pain
peripheral neuropathic pain
what is nociceptive/acute pain
pain from soft tissue damage, infection or inflammation with identifiable cause and location
what is chronic pain
pain lasting more than 6 months
what is neuropathic pain
pain caused by lesion or disease affecting somatosensory system
what are the 2 types of neuropathic pain
Central neuropathic pain (CNS lesions/disease)
Peripheral neuropathic pain (PNS lesions/disease)
why does nociceptive pain usually have identifiable location
because nociceptors are activated at specific tissue sites producing localised signalling
what abnormal sensations can occur in neuropathic pain
dysesthesia (abnormal sensation)
allodynia (pain from non-painful stimuli)
hyperalgesia (increased response to painful stimuli)
what are the typical neuropathic pain symptoms
shooting
stabbing
burning or coldness
pins and needles
itching
chronic anxiety or depression
what are common causes of neuropathic pain
Trigeminal neuralgia
Pain following shingles (postherpetic neuralgia)
Diabetic neuropathy
Phantom limb pain following an amputation.
Multiple sclerosis.
Pain following chemotherapy.
HIV infection.
Alcoholism.
Cancer.
Atypical facial pain.
Pain following a stroke or spinal cord injury
Trauma Post-surgical pain
why can chemotherapy cause neuropathic pain
neurotoxic agents damage peripheral nerves
how can trauma or surgery lead to neuropathic pain
nerve damage → abnormal regeneration and sensitisation
what do nociceptors detect
heat (via TRPVI channels)
cold temps
pressure
chemical signals released during tissue damage (H_, ATP, bradykinin, prostaglandins etc)
tissue damage
how are heat stimuli detected
through TRP channel activation (TRPV1/VR1 and VRL-1)
why does capsaicin (chemical found in peppers that make them spicy) feel hot
it activates TRPV1 receptors, the same channels triggered by heat
what are the 3 sensory fibre types and what do they detect
Aβ – touch/pressure (large, fast)
Aδ – sharp fast pain (medium)
C fibres – slow, dull, burning pain (small, slow)
why is C-fibre pain slow and dull
because C fibres are unmyelinated with very slow conduction speeds (0.5-2m/s)
what do nociceptors do
create electrical signals and send them along nerves to spinal cord
peripheral sensitisation
next few flashcards
what substances are released during tissue injury
bradykinin
histamine
ATP
H+
prostaglandins
5-HT
how do these chemicals cause sensitisation
they lower threshold of nociceptors, making them more responsive
why does NGF contribute to pain
it increases expression of pain receptors (e.g., TRPV1) and sodium channels
what are the 3 main ascending pathways
Spinothalamic tract – fast pain → thalamus
Spinoreticular tract – slow pain → reticular formation
Spinotectal tract – reflex & emotional responses → PAG, limbic system
why is spinothalamic tract important
it conveys location and intensity of pain to cortex
how does spinoreticular pathway affect the pain experience
it contributes to suffering, arousal, and emotional response
where do nociceptive fibres first synapse
in dorsal horn of spinal cord
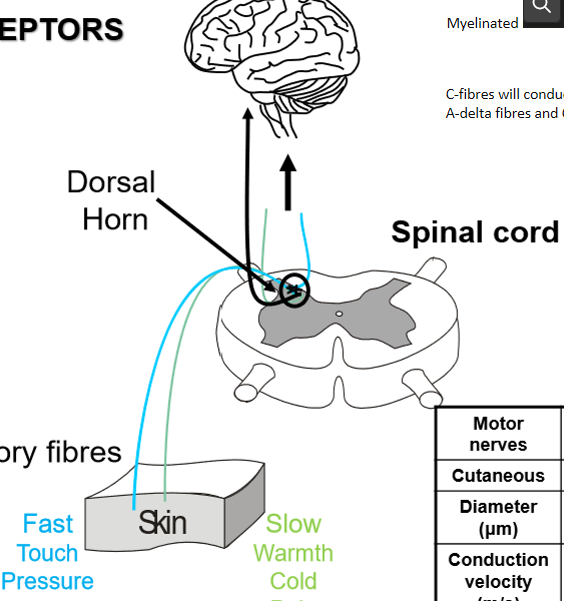
how does dorsal horn modulate pain
through excitatory projection neurons and inhibitory interneurons
why is the dorsal horn a key site for pain modulation
it is where peripheral signals can be amplified or suppressed before reaching brain
what is the Gate Control Theory
pain transmission is modulated by the balance of large-fibre (Aβ) and small-fibre (Aδ/C) inputs
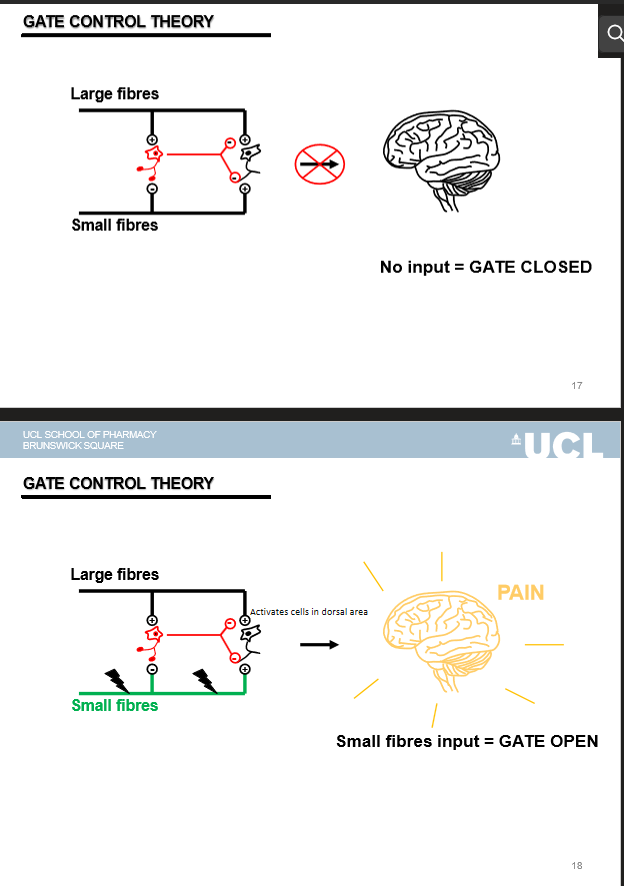
how do large fibres (Aβ) affect the gate
their activation closes the gate → inhibits pain
how do small fibres (Aδ/C) affect the gate
their activation opens the gate → pain transmitted
why does rubbing injury reduce pain
rubbing activates Aβ fibres → closes the gate at the dorsal horn
how does TENS (transcutaneous electrical nerve stimulation) work
by stimulating large fibres to close the gate and inhibit pain signalling
what brain regions control descending pain inhibition
PAG → nucleus raphe magnus → spinal cord
which neurotransmitter mediates descending pain control
serotonin (5-HT) and endogenous opioids
how do descending opioids reduce pain
Inhibit neurotransmitter release from C and Aδ fibres
Activate inhibitory interneurons
Reduce projection neuron firing
why is descending system important
explains how mood, stress, and expectation strongly modify pain perception
how does peripheral sensitisation contribute to neuropathic pain
Cytokines increase nociceptor sensitivity
TRPV1 phosphorylation makes heat receptors hyperactive
Sympathetic sprouting causes abnormal firing
More sodium channels increase spontaneous firing
what is ‘wind-up’ and why does it happen
repetitive C-fibre activation → cumulative depolarisation → progressively increasing pain
how does glutamate contribute to neuropathic pain
Overactivation of NMDA receptors
Reduced glutamate uptake → chronic excitability
how do supraspinal changes worsen neuropathic pain
Cortical remapping enhances pain circuits
5-HT becomes excitatory instead of inhibitory
GABAergic inhibition is reduced
Fewer opioid receptors → opioids become less effective
what pharmacological options treat acute pain
NSAIDs
Opiates
Local anaesthetics
how do NSAIDs reduce pain
by blocking prostaglandin synthesis (less peripheral sensitisation)
how do local anaesthetics block pain
they inhibit voltage-gated sodium channels → block action potentials
why do opioids often fail for neuropathic pain
due to reduced opioid receptor expression and central sensitisation
what types of drugs typically inhibit neuropathic pain
anticonvulsants
antidepressants
topic agents