Amount of substance
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What does percentage of atom economy tell you?
How efficiently atoms in the reactants are used to make the desired products
A high atom economy means most of the atoms from the starting materials end up in the final product with minimal waste
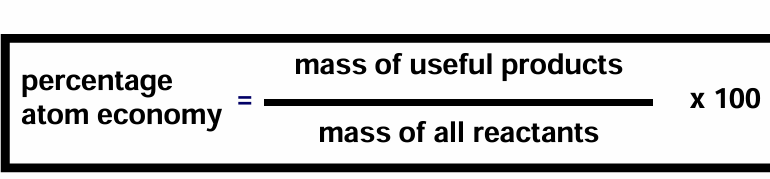
Equation for percentage atom economy
suggest why a conical flask is prefered to a beaker in titration?
Less chance of losing liquid on swirling
Why does repeating the titration make the average titre more reliable?
A single titration could be anomalous
Why does the water used for rinsing have no effect on the accuracy of the titration?
Water is a reagent
How do you calculate percentage yield?
Actual yield/theoretical yield times by a 100
What does avagadro’s constant tell you ?
Number of particles in 1 mol of a substance
What is a titration?
Allows you to determine the concentration of a solution
Using a fixed volume of a solution with a known concentration
How do you carry out a titration?
In this example we are using an acid (unknown solution) and an alkali
Measure an accurate fixed volume of alkali→ using a pipette with a volume of 25cm3
Rinse it with distilled water to remove unwanted chemicals
This may leave water droplets that can dilute the solution, so rinse it again with the alkali.
Put the alkali in a conical flask
Must use an indicator in an acid base titration
Add a few drops of indicator (indicators are weak acids so too much indicator can cause inaccurate results)
Rinse the burette with the acid. Clamp it so its level. Use funnel to pour in acid to the 0 mark
Place the conical flask on a white tile so colour change is more visible
Slowly release the acid into the flask while swirling it
Stop adding acid when there is a colour change to show end point
Read level of acid left in the burette
Subtracting the start volume by final volume=Titre (which is how much acid reacted with the alkali)
Repeat titration until you have 2 concordant results - difference of 0.1 cm3
Calculate mean of concordant results
What is the uncertainty on a burette?
0.05cm 3
When do you use the 2 indicators?
For weak acid and strong base use phenolphthalein
For strong acid and weak base use methyl orange
Why should there be no air bubbles in your burette?
Air bubbles take up space that should be filled with solution
Causing inaccurate volume readings
What is the analyte and what’s the titrant ?
Analyte=unknown solution
Titrant = known solution