Section 3: Glycogen Metabolism and Gluconeogenesis
1/68
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
69 Terms
What are three enzymes involved in glycogen breakdown? In general, what do each do?
1) glycogen phosphorylase → degrades glycogen to glucose-1-phosphate
2) glycogen debranching enzyme → acts as a glucosyltransferase
3) phosphoglucomutase → interconverts glucose-1-phosphate and glucose-6-phosphate (second intermediate in glycolysis)
What are the major roles of glycogen and starch in terms of metabolism?
function to stockpile glucose for later metabolic use
need a constant supply around 5mM in blood to all tissues
Why do we need a constant supply of glucose?
essential for the brain and red blood cells
they both depend on glucose as an energy source
Where does the breakdown of glycogen to form glucose happen? (2)
1) skeletal muscle
2) liver
In terms of glycogen breakdown or synthesis what happens when glucose concentration in blood are high?
glycogen synthesis accelerates
How long can the liver’s glycogen storage supply the brain with glucose?
glycogen stores in the liver have only enough glucose to supply the brain for ½ day
In terms of metabolism what happens when we are in fasting conditions?
glucose needs are met from gluconeogenesis (making of glucose) from non-carbohydrate precursors (ex: amino acids)
What is McArdle’s disease?
deficiency in glycogen phosphorylase (glycogen break down enzyme)
glycolysis does not occur at a sufficient rate proportional to demand for ATP
results in painful muscle cramps on exertion
What are three characteristics of glucose-6-phosphate
1) key branch point in terms of metabolism (can be broken down further into pyruvate continuing glycolysis, could enter PPP, or it can be broken down further into glucose for gluconeogenesis) → allows the cell to save several intermediates that would otherwise require additional energy
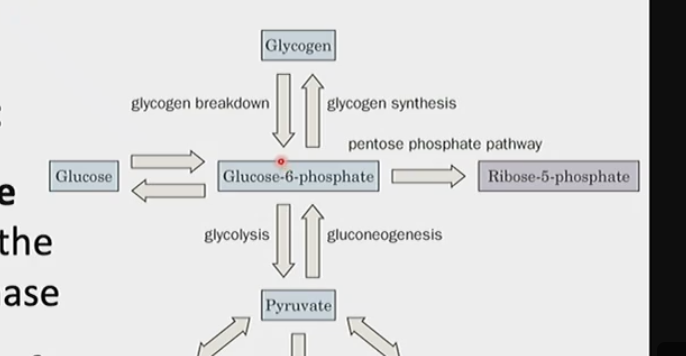
2) derived from free glucose in glycolysis through the action of hexokinase (first step)
3) product of glycogen breakdown or gluconeogenesis
Can you reverse the enzymes used to break down glycogen in order to make glycogen?
no
have four separate enzyme involved in glycogen synthesis from glucose-6-phosphate
What is glycogen?
storage form of glucose that is a branched polymer and occurs as intracellular granules that are spheroidal
contains alpha-1,4 linked D-glucose
contains alpha-1,6 linked branches every 8-14 residues
each spheroidal molecule contains up to 120,000 glucose units
When glycogen is stored in skeletal muscle and the liver, can it be broken down and utilized by other tissues?
glycogen can only be broken down and mobilized to different tissues in the liver, if glycogen is present in the muscle then its breakdown into glucose will primarily be used by the muscle
In order for glycogen from the liver to be mobilized to different tissues describe the breakdown.
glycogen → glucose-1-phosphate → glucose-6-phosphate → glucose
Describe the number of reducing ends to non-reducing ends in glycogen? Why is it beneficial?
has only one reducing end with multiple non-reducing ends
enzymes (glycogen phosphorylases) used to break it down utilize the non-reducing end to break it down, allowing for more glucose units to be released simultaneously and thus permits rapid glucose mobilization (specifically in liver cells)
What percent of glycogen by weight is found in muscle versus liver cells?
muscle → 1-2% glycogen by weight
liver cells → 10% glycogen by weight
What enzymes are found in muscle and liver cells?
both contain enzymes that catalyze glycogen synthesis and degradation, as well as proteins that regulate these processes
all found within glycogen granules
How does glycogen phosphorylase work?
breaks down glycogen and adds a phosphate to the glucose units to release glucose-1-phosphate
only works if the units are least 5 units way from a branch point (requires space to be active)
only works on non-reducing ends of glycogen
What are some characteristics of glycogen phosphorylase?
degrades glycogen to G1P → dimer of identical 842-residue subunits
catalyzes the rate-controlling step in glycogen breakdown
In general what are the two mechanisms that regulate glycogen phosphorylase?
1) allosteric interactions
2) covalent modification (phosphorylation and de-phosphorylation)
extremely sensitive regulation process
What are the three allosteric inhibitors of glycogen phosphorylase?
1) ATP
2) glucose-6-phosphate
3) glucose
What is the one allosteric activator of glycogen phosphorylase?
1) AMP
Explain how covalent modification regulated glycogen phosphorylase?
when the enzyme is phosphorylated OR dephosphorylated this impacts how allosteric inhibitors/activators interact with it
Describe the active site of glycogen phosphorylase.
small opening on the surface
connects/links to the glycogen storage unit
can accommodate four or five sugar residues in a chain and is too narrow to admit branched oligosaccharides
What cofactor is required for glycogen phosphorylase to work?
pyridoxal-5’-phosphate (PLP)
vitamin B6 derivate
required for activity
What are the two different conformational changes of glycogen phosphorylase? Describe each one.
1) T-state (inactive): active site is buried and thus this state has a low affinity for its substrates
2) R-state (active): has an accessible catalytic site that has a high affinity for substrates
Describe how AMP influences conformational changes within glycogen phosphorylase.
AMP promotes the conformational shift of glycogen phosphorylase from T → R
binds to the R state of the enzyme at its allosteric effector site and this binding disorders a loop that would have otherwise blocked the active site
increases access of substrate to active site
Describe how ATP influences conformational changes within glycogen phosphorylase.
inhibits the phosphorylase shift from inactive (T) → active (R)
binds to the T state of the enzyme at its allosteric effector site
How does the glycogen debranching enzyme work?
breaks down/removed glycogen’s branches (limit branch) by acting as an alpha-1,4 transglycosylase/glycosyltransferase of three glucose subunits off the branch
moves the three units from the branch and extends the already existing non-reducing end
makes these branches accessible to glycogen phosphorylase
very first glucose unit in the branch linked by an alpha-1,6 linkage is released as pure glucose via hydrolysis
makes up about 10% of what is released during glycogenolysis
How many active sites are found in glycogen debranching enzyme?
two
What are the two active sites of glycogen debranching enzyme? What are each of their functions?
1) active site for transferase activity
2) active site for alpha-1,6 glucosidase reaction → to release pure glucose
two independent active sites improves debranching efficiency
Is the rate of glycogen phosphorylase higher or lower than the rate of glycogen debranching activity?
glycogen phosphorylase occurs at a higher rate compared to glycogen debranching rate
overall degradation stalls while debranching is taking place
explains why a muscle can sustain its maximum exertion for only a few seconds
How does the phosphoglucomutase enzyme work?
enzyme that interconverts glucose-1-phosphate → glucose-6-phosphate in the cytosol
intermediate step: phosphoryl group is transferred from the active phosphoenzyme to glucose-1-phosphate → glucose-1,6-bisphosphate → glucose-6-phosphate
Why does glucose-6-phosphate have to get further broken down into glucose when we are talking about glucose mobilization in the liver?
glucose-6-phosphate cannot pass through the cell membrane by itself therefore it has to be hydrolyzed by glucose-6-phosphatase (G6Pase) in order to turn into glucose + Pi and then enter the tissue
Describe the events that must occur in order for glucose-6-phosphate to get hydrolyzed into glucose and enter tissues.
glucose-6-phosphate is produced in the cytosol whereas the glucose-6-phosphatase is found in the ER
glucose-6-phosphate is imported into the ER by glucose-6-phosphate translocase before it can hydrolyzed
resulting glucose and Pi are returned to the cytosol via transport proteins
What is type I glycogen storage disease?
defect in the components of glucose-6-phosphate hydrolysis system to glucose
Do liver and muscle cells use the same glucose transporting system?
no
Describe how glucose leaves liver and muscle cells to go to other tissues.
liver cells
leaves via GLUT2
muscle cells (and other tissues)
lack glucose-6-phosphatase and thus they retain their glucose-6-phosphate (which makes sense because to begin with they don’t mobilize their glucose)
What are the three enzymes involved in glycogen synthesis?
1) UDP-glucose pyrophosphorylase
2) glycogen synthase
3) glycogen branching enzyme
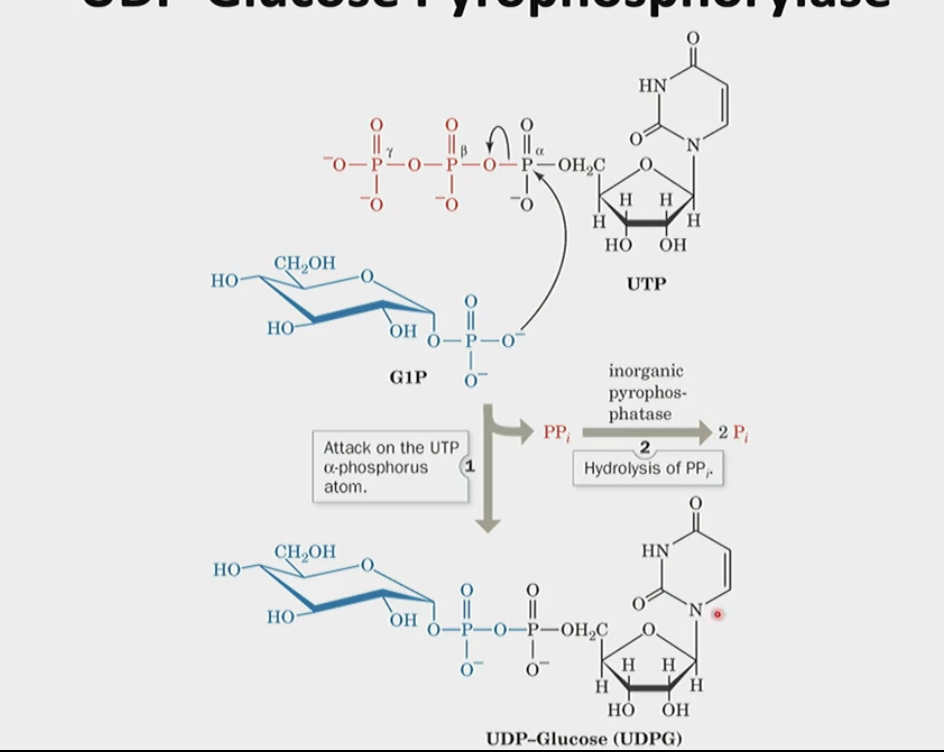
How does UDP-glucose pyrophosphorylase work?
activates glucosyl units to help extend existing glycogen subunits
Briefly describe the steps involved in glycogen synthesis based off of this image.
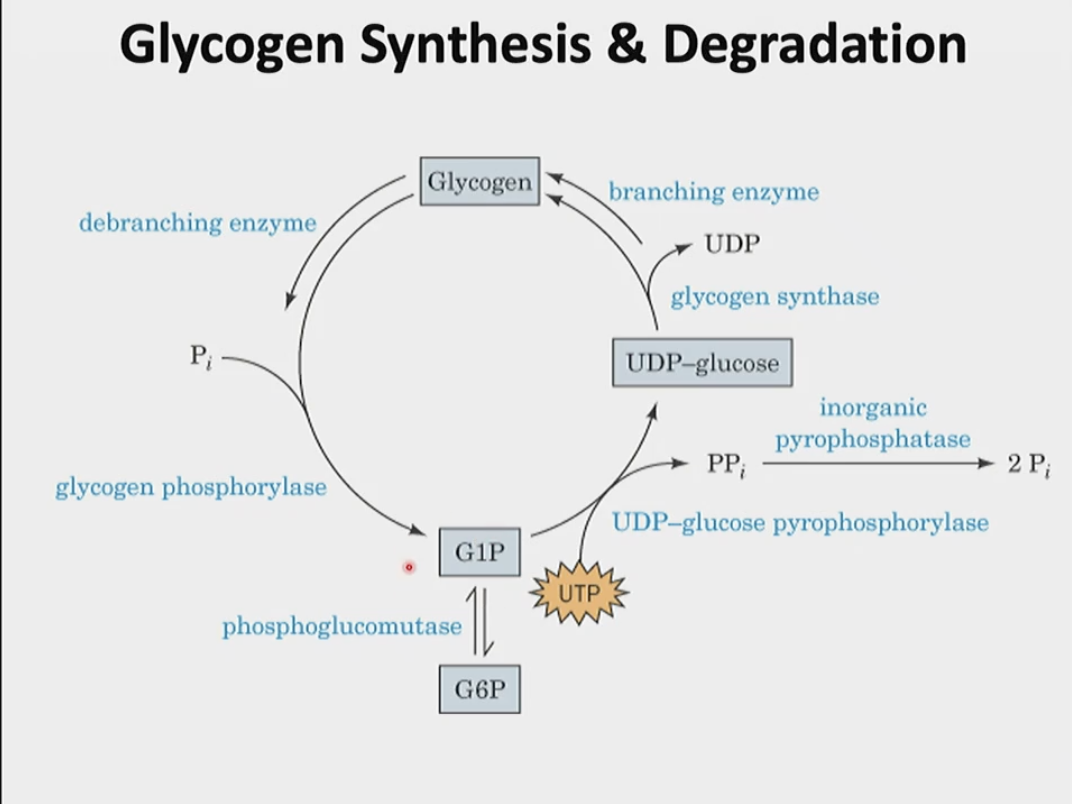
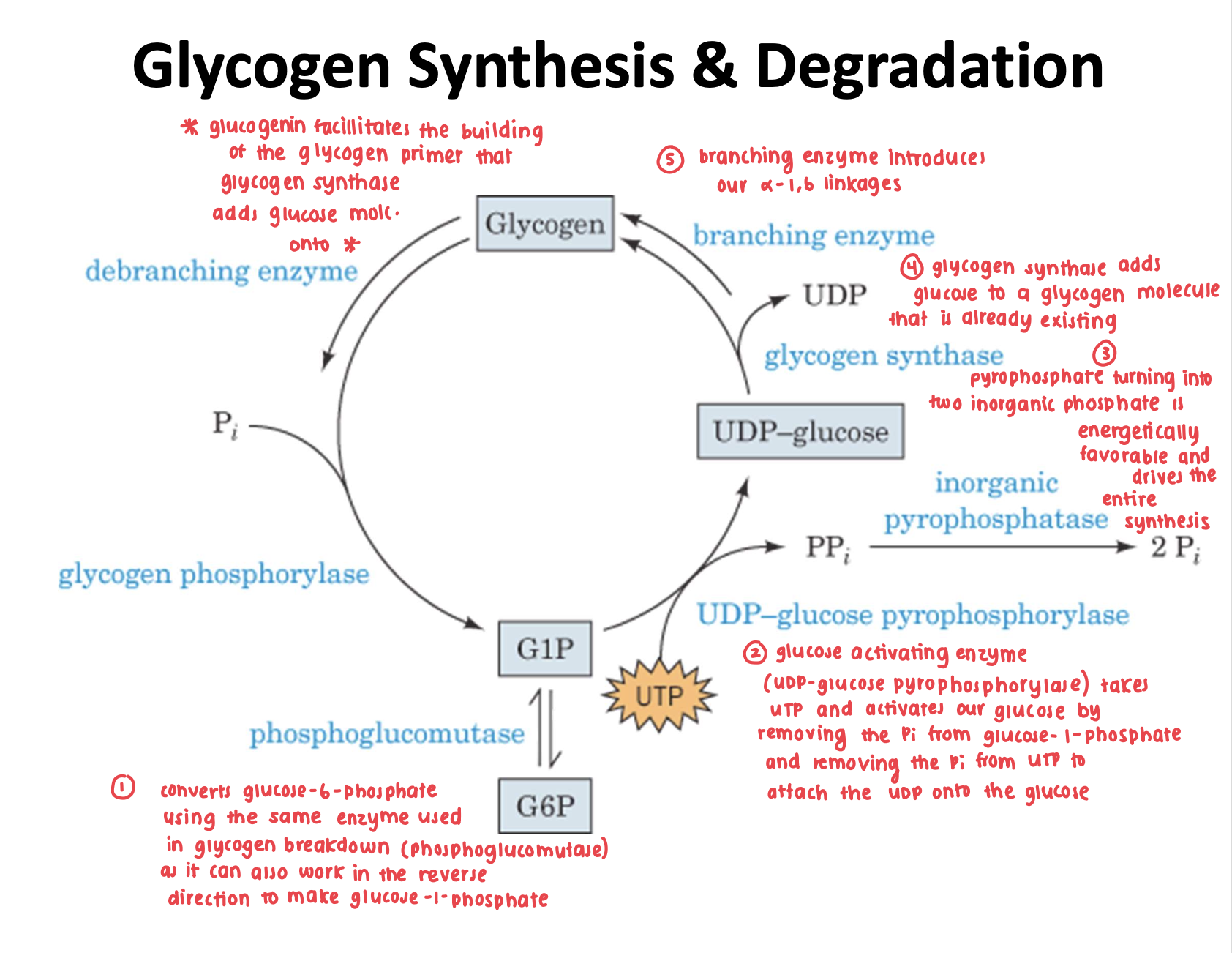
Is glycogen synthesis starting from glucose-1-phosphate favorable?
no, it is thermodynamically unfavorable
driving force/kept favorable as it is coupled with the reaction in which pyrophosphate is degraded into two inorganic phosphate molecules
How does the UDP-glucose pyrophosphorylase enzyme work?
activates glucosyl units from glucose-1-phosphate into uridine diphosphate glucose (UDPG)
UDPG is now activated and can be added to a growing glycogen chain with the help of another enzyme
this step itself has a delta G around 0 (thermodynamically not favorable) therefore it is couple with pyrophosphate → 2 inorganic phosphate (has a negative delta G, making the overall negative/favorable)
How does the glycogen synthase enzyme work?
extends the glycogen chain by transferring UDPG to the C4-OH group on one of the glycogen’s non-reducing ends to form an alpha-1,4 glycosidic bond
overall reaction is spontaneous
When UTP is transformed into UDP during glycogen synthesis how does the UTP get replenished?
nucleoside diphosphate kinase uses an ATP to do a phosphoryl-transfer reaction to help replenish UTP
UTP consumption is energetically equivalent to ATP consumption (explains why activating glucose is costs us energetically)
Since glycogen synthase can only extend already existing glycogen chains, what helps facilitate the formation of the initial chain?
glycogenin
Explain how glycogenin works.
acts as a glycosyltransferase as it attaches glucose residues (donated by UDPG) to the OH group of it’s own Tyr194, essentially building the primer glycogen molecule that glycogen synthase can extend
extends the glucose chain by up to 7 glucose residues donated by UDPG
How does the glycogen branching enzyme (amylo-(1,4→1,6)-transglycosylase) work?
glycogen branching enzyme transfers 7 residue glycogen segments from the chain end and links them to the C6-OH of another glucose residue yielding an alpha-1,6 linkage
distinct from glycogen synthase and glycogen debranching enzyme
branching pattern is optimized for efficient storage and mobilization of glucose
What are two rules regarding how the glycogen branching enzyme works?
1) each transferred segment must come from a chain at least 11 residues long
2) each branch point must be at least 4 residues away from other branch points (can be on the fourth one so technically three in-between)
What are two ways that glycogen phosphorylase and glycogen synthase activity are controlled?
1) allosteric control
2) control by covalent modification
What are the different ways that glycogen phosphorylase and glycogen synthase can be controlled through covalent modification? (3)
1) glycogen phosphorylase activated by phosphorylation
2) phosphorylase kinase activated by phosphorylation and by Ca2+
3) phosphoprotein phosphatase-1 inhibited by phosphoprotein inhibitor-1
What happens to glycogen phosphorylase and glycogen synthase when we have low [ATP], low [glucose-6-phosphate], or high [AMP]?
means that we have a high demand for ATP and thus need to break down glycogen stores
glycogen phosphorylase is stimulated
glycogen synthase is inhibited
What happens when we have high levels of [ATP] and [G6P]?
glycogen synthesis is favored
Explain how glycogen phosphorylase is activated by phosphorylation.
two versions of glycogen, form a and form b
glycogen phosphorylase form a = more active
glycogen phosphorylase form b = less active
What three enzymes regulate the phosphorylation and de-phosphorylation of glycogen phosphorylase.
1) phosphorylase kinase
2) protein kinase A
3) phosphoprotein phosphatase-1
Describe the regulatory mechanism behind phosphorylase kinase and how it impacts glycogen phosphorylase.
in it’s active form phosphorylase kinase can help bring glycogen phosphorylase into it’s active “a” form and directly impact it
direct regulatory of glycogen phosphorylase
Describe the regulatory mechanism behind protein kinase A and how it impacts glycogen phosphorylase.
Protein kinase A → activates phosphorylase kinase so that it is in its active form by phosphorylating its alpha and beta subunits (beta and c-terminal segment move aside, stopping the block of the gamma subunit, allowing for catalytic activity)
active phosphorylase kinase → activates glycogen phosphorylase form a
Protein kinase A indirectly regulates the activation of glycogen phosphorylase
Describe the regulatory mechanism behind phosphoprotein phosphatase-1 and how it impacts glycogen phosphorylase.
can regulate directly and indirectly
directly → inactivates glycogen phosphorylase a to glycogen phosphorylase b
indirectly → inactivates phosphorylase kinase by de-phosphorylating the alpha and beta subunits, thus decreasing the activation of glycogen phosphorylase
Describe the structure of phosphorylase kinase.
phosphorylase kinase contains four non-identical subunits (alpha, beta, gamma, and delta)
alpha, beta, and delta subunits have regulatory functions
gamma contains the catalytic site
full catalytic activity of phosphorylase kinase in its less active form is prevented by an autoinhibitory C-terminal segment and the beta subunit as they block the gamma subunit
Along with phosphorylation from protein kinase A, what other aspect can regulate phosphorylase kinase?
Ca+
Describe how calcium regulates phosphorylase kinase.
Ca2+ can bind to the delta subunit, and depending on the concentration this could active the enzyme
the delta subunit is similar to calmodulin (also binds to Ca2+)
Ca2+ concentrations as low as 10^-7M can activate phosphorylase kinase
What regulates phosphoprotein phosphatase-1?
Is the regulation of phosphoprotein phosphatase-1 the same in the muscle and the liver?
no
Based on this image describe the regulation of phosphoprotein phosphatase-1 in muscle cells.
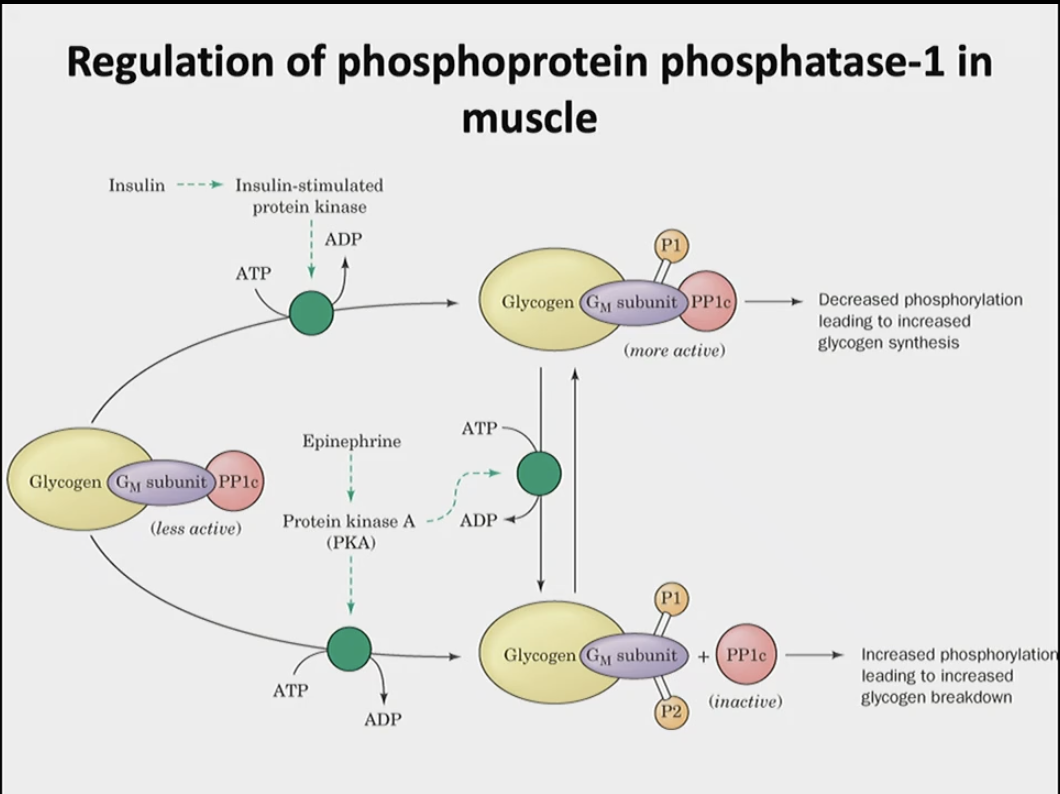
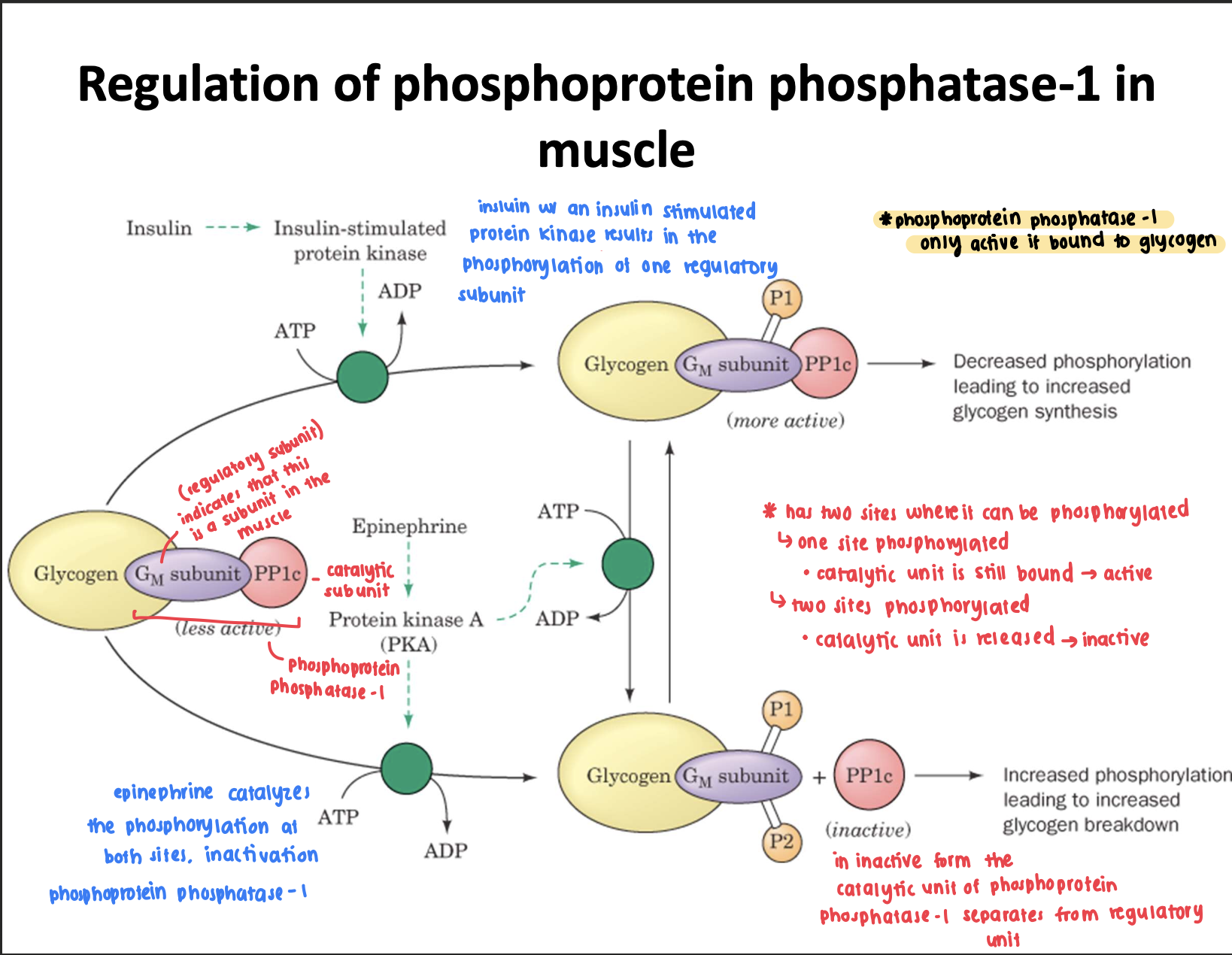
In general describe how phosphoprotein phosphatase-1 is regulated in muscle, aka when is it active when is it not active?
active: when the catalytic subunit is bound to glycogen through the glycogen-binding regulatory subunit (GM)
binding of catalytic subunit is dependent on the phosphorylation state of GM
phosphorylated in both sites → disconnected → inactive
phosphorylated in one site → connected → active
In general describe how phosphoprotein phosphatase-1 is regulated in the liver?
phosphoprotein phosphatase-1 is bound to glycogen through a glycogen binding subunit named GL
however GL is not subject to control via phosphorylation compared to the GM
What are two things that regulate the activation/inactivation of phosphoprotein phosphatase-1? Briefly describe how they work and their impact on glycogen synthesis or break down.
1) insulin → insulin-stimulated protein kinase → phosphorylates one site on the regulatory unit → activates phosphoprotein phosphatase-1 → increases glycogen synthesis (as it inhibits glycogen phosphorylase)
2) epinephrine → protein kinase A (can phosphorylate site one or site 2 or both)→ phosphorylates two sites on the regulatory unit → inactivates phosphoprotein phosphatase-1 as the catalytic subunit detaches → increase in glycogen breakdown
Besides the regulation through insulin and epinephrine, what is another thing that regulates phosphoprotein phosphatase-1?
phosphoprotein phosphatase inhibitor 1 → inhibits phosphoprotein phosphatase-1
What activates phosphoprotein phosphatase inhibitor 1?
PKA
makes sense bc through epinephrine that utilizes PKA, it also inhibits phosphoprotein phosphatase-1
What deactivates phosphoprotein phosphatase inhibitor 1?
phosphoprotein phosphatase-1