Peptides and Peptide Bond
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
45 Terms
formed by the covalent interaction of two amino acids
A polypeptide
A molecule of water is eliminated for each peptide bond formed, what is the product called
dipeptide
what is the remaining portion of the AA in the peptide called
amino acid residue
What terminal ends are available for further reactions in peptide bonding
N-terminal and C-terminal
what catalyzes the condensation reaction of peptides
ribosomes
which type of molecules transport amino acids in to the ribosome
tRNA
Where are amino acids added successively of a growing peptide
the carboxyl terminal end
what is the primary structure of the protein
amino acid sequence
who analyzed the geometry and dimensions of peptide bonds in crystal structures of molecules containing one or a few peptide bonds
Linus Pauling and Robert Corey
C-N bond length is what percent shorter than found in usual amines
10%
in relevance to the planar structure of peptide bonds, why is the peptide bond short
because the C-N bond has some double bond character (40%) due to resonance with the C=O group
what are all peptide bonds approximately
coplanar
what reduces the degrees of freedom during folding in relation to the planar structure of peptide bonds
the rigidity of the peptide bond
what are the three main torsion angles of a polypeptide backbone
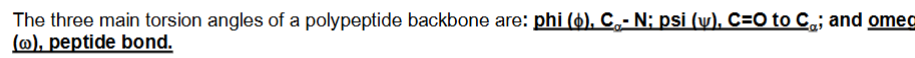
What degrees does the planarity of the peptide bond restrict w to in nearly all the main chain peptide bonds
180 (trans)
in rare cases, for a cis peptide bond which usually involves proline
w=0 degrees
in peptide bonds for the cis and trans configuration, what two bonds are nearly coplanar
C=O and N-H
what configuration is the peptide bond nearly always in because of it being favorable over cis
the peptide bond
what side are alpha C atoms on in C-N peptide bonds in the trans isomer
opposite sides
what side are alpha C atoms on in C-N peptide bonds in the cis isomer
same side
what is significant and found between functional groups attached to the alpha carbon in peptide bonds
steric hinderance is greater in the cis configuration
In relation to peptide bonds involving proline, what allows both the cis and trans configurations to have nearly equivalent energies , thus allowing proline to be found in the cis configuration
the cyclic nature
in relation to the formation and stability of peptide bonds- true or false formation of a peptide bond is not favored thermodynamically
true
in relation to the formation and stability of peptide bonds- formation of a peptide bond is not favored thermodynamically, what reaction is favored instead?
Hydrolysis of the peptide bond
what type of energy is used during translation
ATP
what are short peptides of a few residues called
oligopeptides
what are longer chain peptides of a few residues called
polypeptides
what are known as very long chains of polypeptides folded in to regular structure
proteins
what does the term polypeptide refer to
a chain of amino acids
what does the term protein refer to
the chain of amino acids after it folds properly and is (in some cases ) modified
true or false : proteins may consist of more than one polypeptide chain ( multimer)
true
what is the average molecular weight of an amino acid
138
when accounting for the abundance of AA’s in known proteins, the molecular weight of an average AA in a protein is about
128
why is the avg weight of an amino acid reside in a protein 110
because peptide bond formation removes a water molecule ( which has a molecular weight of 18)
how do you estimate the number of residues in a protein
by dividing the molecular weight by 110
true or false: the number of unique sequence possibilities is enormous in peptides
true
How many sequences do organisms typically rely on so necessary function will determine which sequences are constructed
30,000-35,000
True or False: Proteins ( polypeptides) contain an equal distribution ogf amino acids
False - they do not contain an equal distribution
Each of the amino acids, joined together by peptide bonds, in proteins (or polypeptides) have different properties determined by ____
R group side chains
what are the different properites that can make up R group side chains
acidic, basic, neutral, hydrophobic
What do amino acid side-chains direct
the folding of the nascent polypeptide into a functional protein and stabilize its final conformation
some proteins contain chemical groups other than amino acids, called conjugated proteins taht contain permenantly associated medical components… what is the non amino acid part called
prosthetic group
some proteins contain chemical groups other than amino acids, what id the prosthetic group for lipoproteins
lipids
some proteins contain chemical groups other than amino acids, what is the prosthetic group for glycoproteins
sugars
some proteins contain chemical groups other than amino acids, what is the prosthetic group for metalloproteins
specific metals