CH. 4 Nomenclature and Conformations of Alkanes and Cycloalkanes
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
Cycolalkanes
rings with only single bonds
Shapes of Alkanes
Alkanes contains sp3 hybridized carbons and are tetrahedral
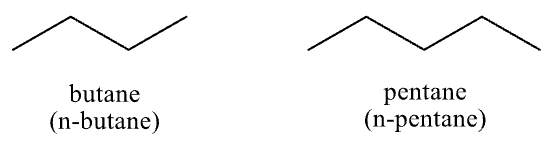
straight chain or unbranched alkanes
normal or n-alkanes
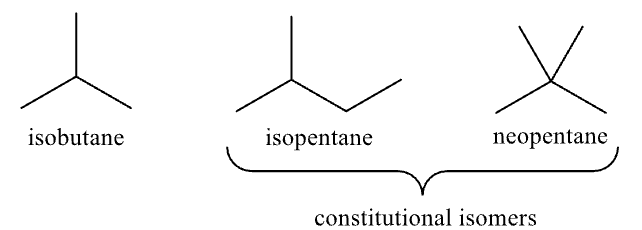
Branched Alkanes
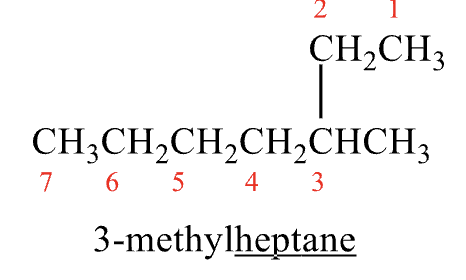
Rules for Naming #1
locate the parent (longest) chain
It is not always in a straight line
Rules for Naming #2
Number the atoms in the main chain; start at the end closest to the branching
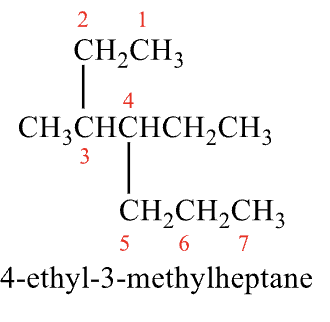
Rules for Naming #3
Identify and number the substituents (groups)
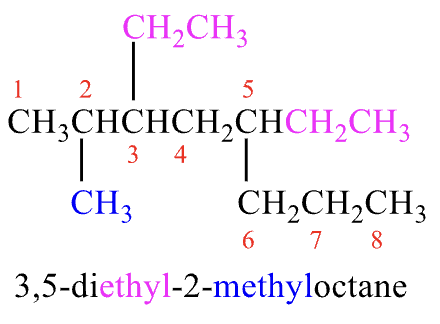
Rules for Naming #4
two or more substituents: list in alphabetical order
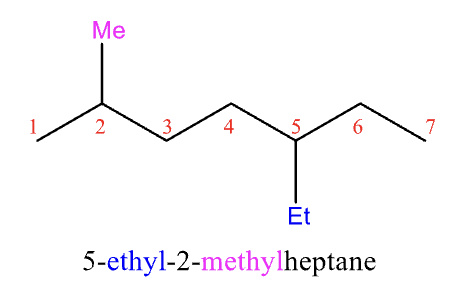
Rules for Naming #5
substituents are the same carbon: use number twice
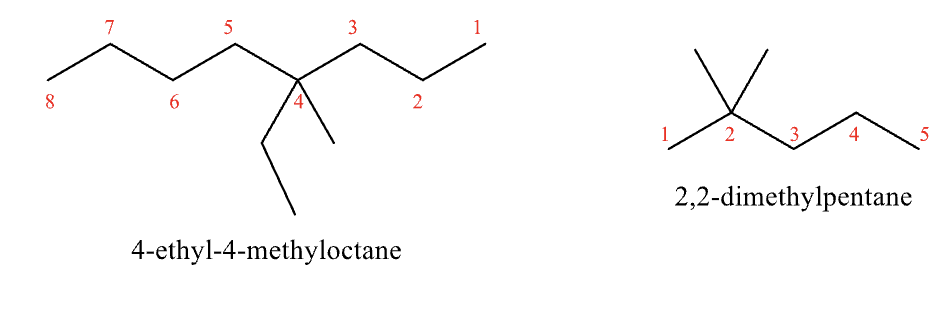
Rules for Naming #6
if there are multiple substituents that are the same use the prefix di, tri, tetra, etc.
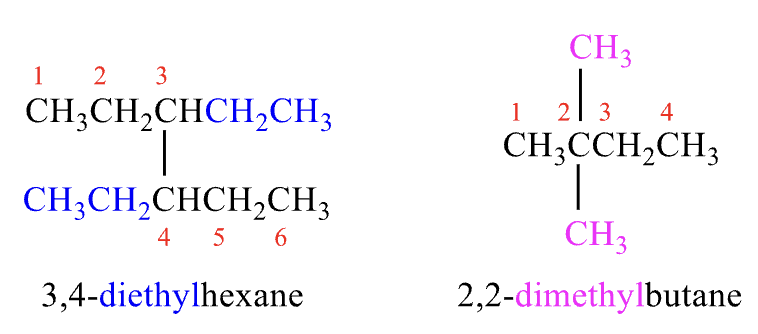
Rules for Naming #7
if there are two chains of equal length, choose the one with the larger number for branching
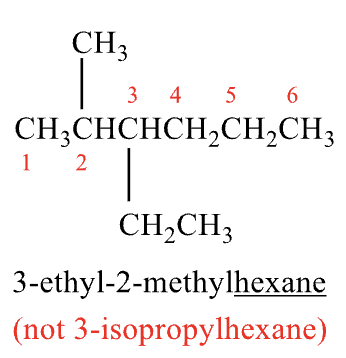
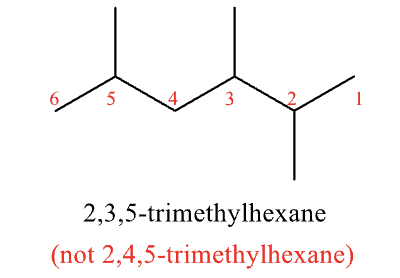
Rules for Naming #8
when branching occurs at equal distance from either end, choose the name that gives the lower number at the point of difference
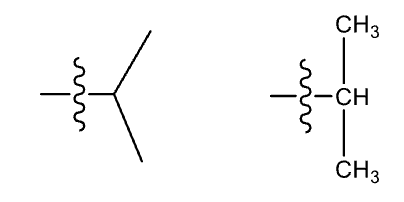
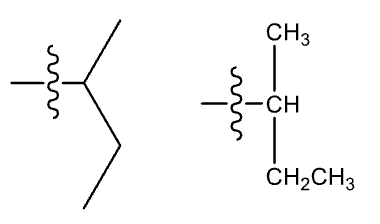
Isopropyl group (iPr)
non-systematic
sec-Butyl group (sec-Bu)
non-systematic
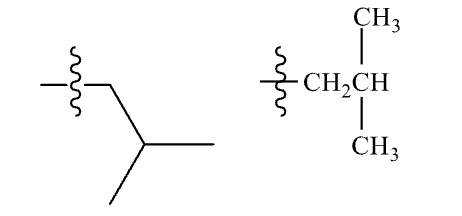
Isobutyl group
non-systematic
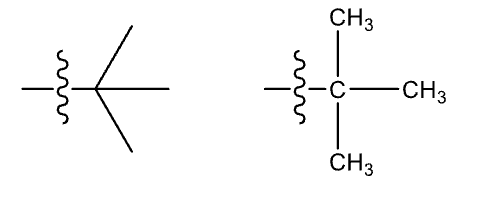
Tertiary-butyl group (t-Bu or tert-Bu)
non-systematic
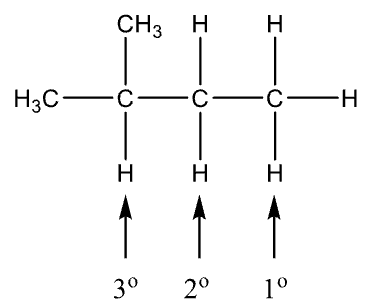
Classification of Hydrogen Atoms
1o hydrogen is attached to a 1o carbon
2o hydrogen is attached to 2o carbon
3o hydrogen is attached to 3o
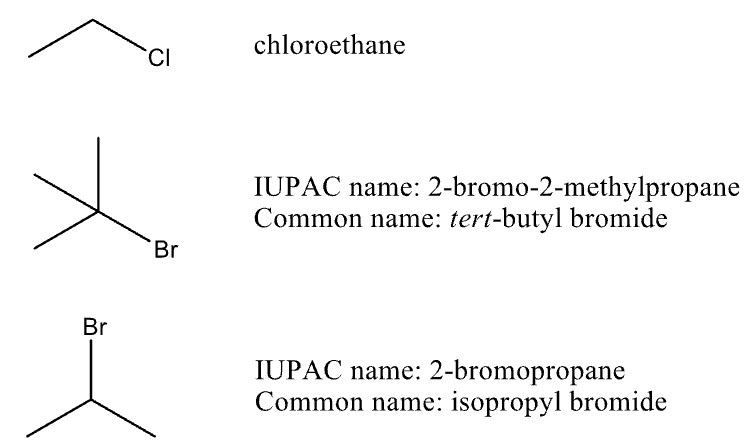
Naming Alkyl Halides
drop the “-ine” and add “-o”
Chlorine→ chloro
Bromine→ bromo
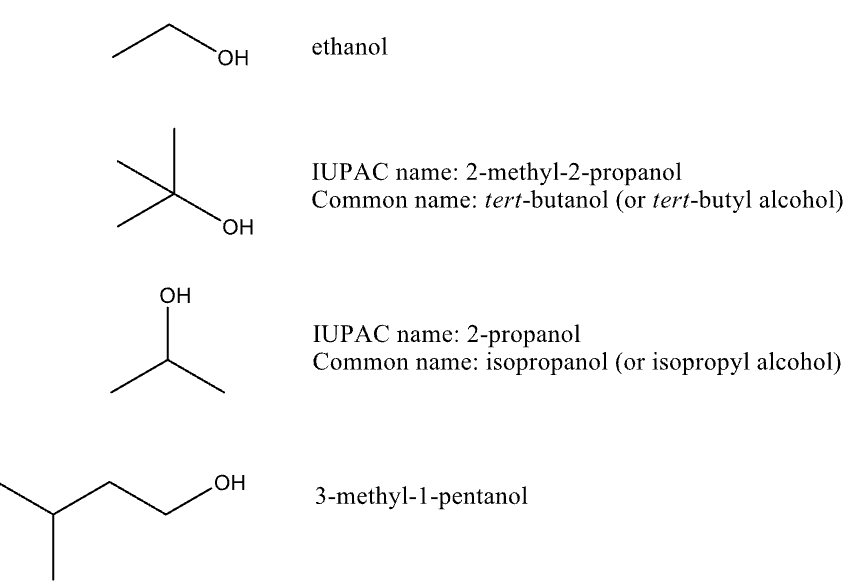
Naming Alcohols
drop “-e” and add “-ol” suffix
Glycols and diols (IUPAC)
alcohols with two hydroxyl groups

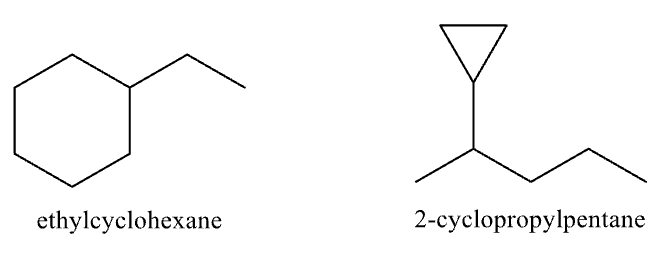
Naming Cycloalkanes Rule #1
Find the parent chain
If the number of carbon atoms in the ring is equal to or greater than the number of carbons in the substituents, the compound is named as an alkyl-substituted cycloalkane
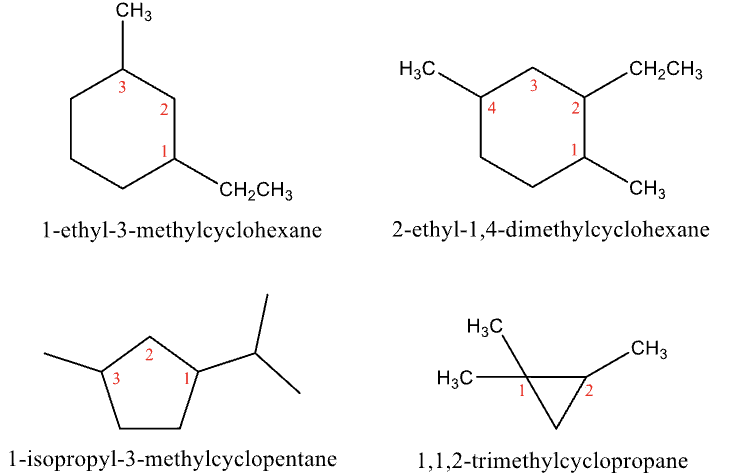
Naming Cycloalkanes Rule #2
Number the substituents
Start #1 at the substituent that gives the lowest possible number then put in alphabetical order
Naming Cycloalkanes Rule #3
If more than one ring is attached, name as a cycloalkyl-substituted alkane
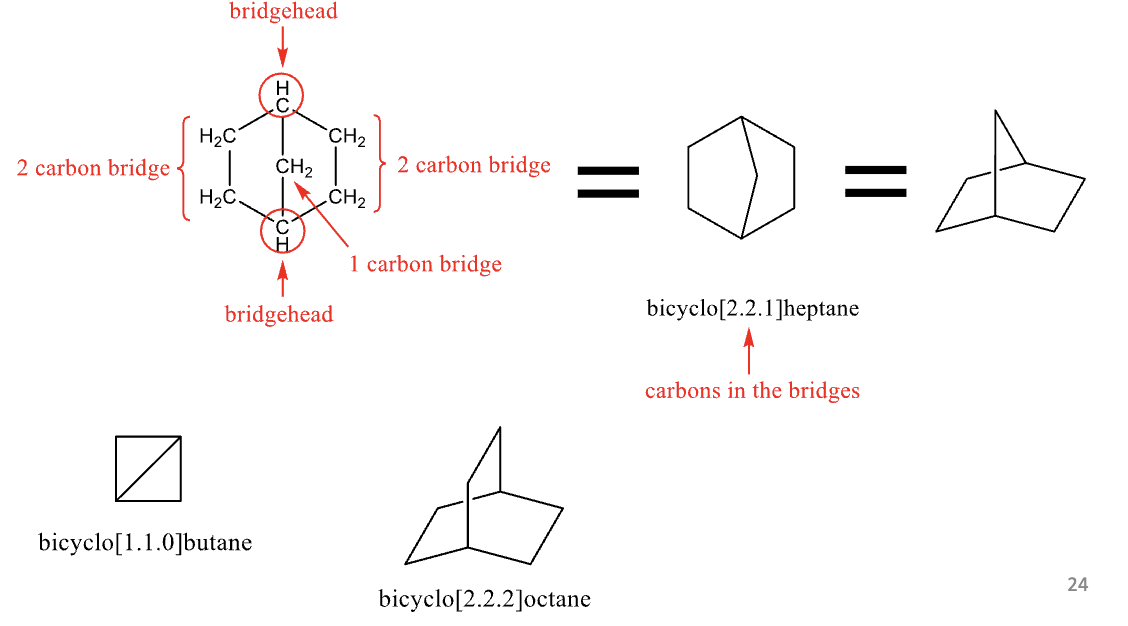
Bicyclic Compounds
fused ring structures
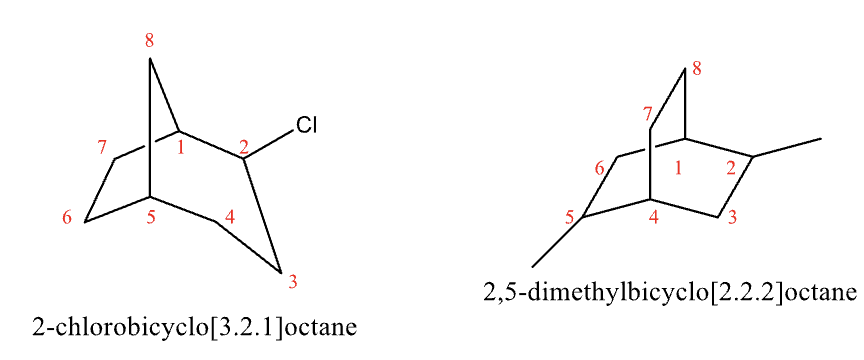
With substituents, start numbering at bridgehead then go around the largest ring first
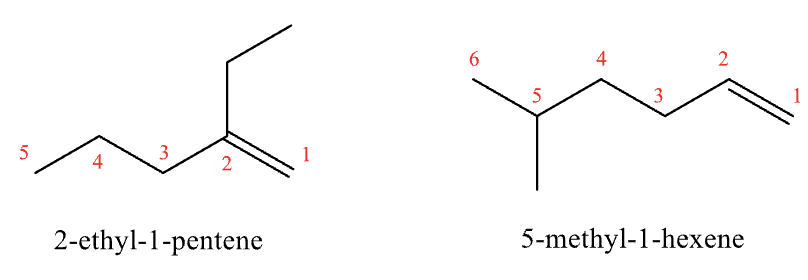
Naming Alkenes and Cycloalkenes Rules #1 and #2
#1: find the longest chain that contains the double bond, drop -ane and use the -ene suffix
#2: both carbons of the double bond must be included in the numbering
Naming Alkenes and Cycloalkenes Rule #3
number at the end closest to the double bond; substituents are not the highest priority
If equal distance from either end, start at the end closest to branching
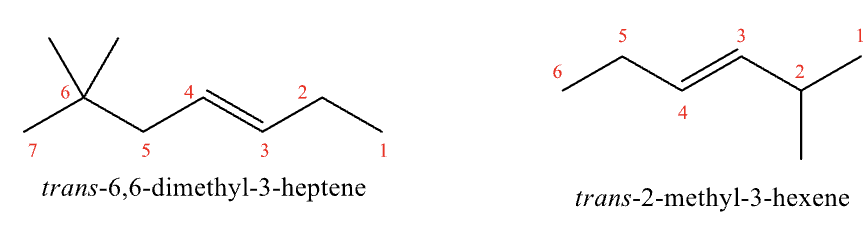
Naming Alkenes and Cycloalkenes Rule #4
For cycloalkenes: the double bond is #1 and #2 then number the rest of the substituents giving the lowest number possible
Naming Alkenes and Cycloalkenes Rule #5
For double bond and alcohol, name as a alcohol
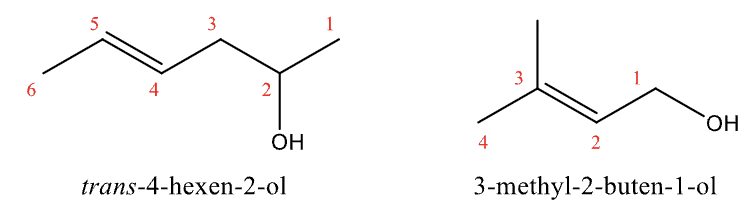
Naming Alkenes and Cycloalkenes Rule #6
alkenes can be groups on other molecules
Naming Alkenes and Cycloalkenes Rule #7
Cis- and trans- prefix can be used when you have two substituents (not hydrogen) on opposite end of the double bond
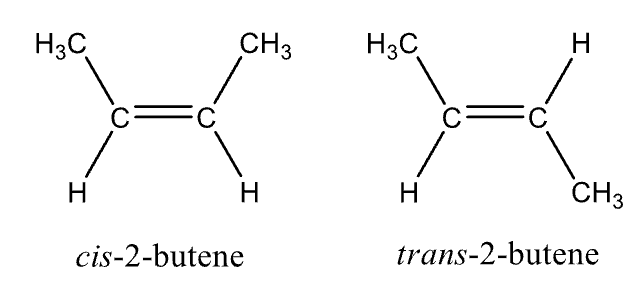
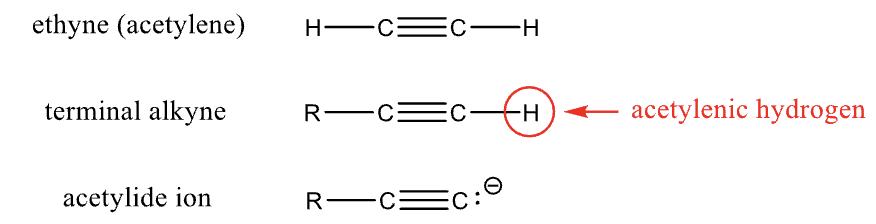
Naming Alkynes
drop -ane and use the -yne suffix
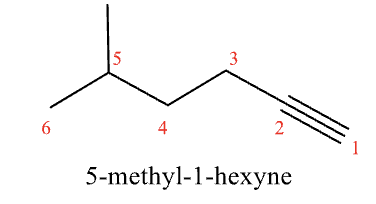
Named as alcohols if they contain -OH
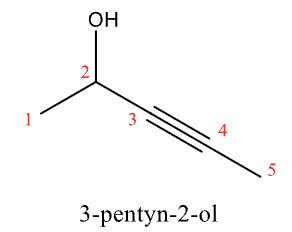
Terminal alkyne
known as a mono-substituted acetylene
Physical Properties of Alkanes
Boiling point of unbranched alkanes increase with the molecular weight
Branching decreases surface area and decrease boing point
Density- alkanes and cycloalkanes have very low densities and float on water
Alkanes and cycloalkanes are insoluble in water
Sigma Bond and Bond Rotation
Groups bonded by sigma bonds have free rotation about the bond
The temporary shape is known as conformation
Each possible structure is a conformer
The Newman projection and Sawhorse formulas are useful for looking at conformations