Biological molecules
1/49
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
50 Terms
Describe how a molecule of beta glucose is different from a molecule of alpha glucose. (2 marks)
Hydroxyl group on carbon 1 is in a different position
In alpha glucose it is below the ring, in beta glucose it is above the ring
Glycogen and cellulose are both carbohydrates. Describe two differences between the structure of a cellulose molecule and a glycogen molecule. (2 marks)
Cellulose is made up of β-glucose monomers and glycogen is made up of α-glucose
Cellulose molecule has straight chain and glycogen is branched
Glycogen has 1,4 and 1,6 glycosidic bonds and cellulose has only 1,4 glycosidic bonds
A covering of lignin protects cellulose from enzyme attack . Use your knowledge of the way in which enzymes work to explain why cellulose-digesting enzymes do not digest lignin. (2 marks)
Enzymes are specific
Shape of lignin molecules will not fit active site (of enzyme)
Describe the structure of a cellulose molecule and explain how cellulose is adapted for its function in cells. (6 marks)
made from β-glucose
joined by condensation, glycosidic bond
hydrogen bonds linking long straight chains
cellulose fibres makes cell walls strong
can resist osmotic pressure
bond difficult to break; resists digestion / enzymes
Explain how cellulose molecules are adapted for their function in plant cells (3 marks)
Long and straight chains
Become linked together by many hydrogen bonds to form microfibrils
Provide strength to the cell wall
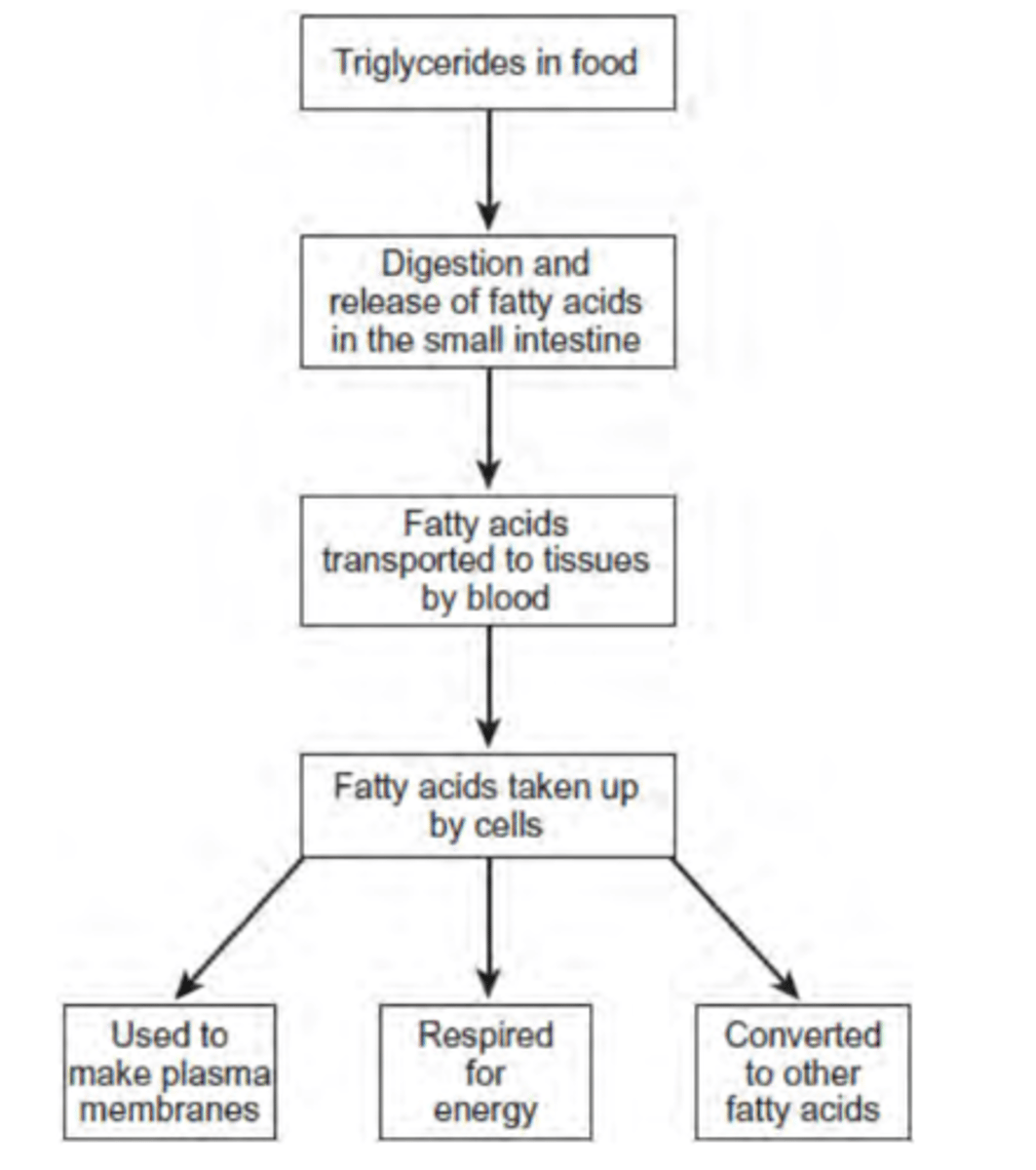
Use the information in the figure to explain two ways in which fatty acids are important in the formation of new cells. (4 marks)
1. Fatty acids used to make phospholipids; Phospholipids in membranes; More phospholipids more membranes made;
2. Fatty acids respired to release energy; More triglycerides more energy released; Energy used for cell production
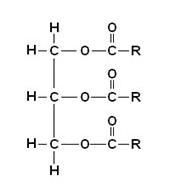
Draw a diagram to show the structure of a triglyceride molecule. (2 marks)
Define the quaternary structure of a protein. (1 mark)
Multiple polypeptide chains held together by hydrogen bonds and ionic bonds
Explain how two enzymes with different amino acid sequences can catalyse the same reaction. (2 marks)
Both active sites have similar tertiary structures so can form enzyme-substrate complexes with the same substrates
Explain how a change in the primary structure of a globular protein may result in a different three-dimensional structure. (3 marks)
Sequence of amino acids change
Tertiary structure folds in a different way
As bonds form in different places
Explain how the structure of fibrous proteins is related to their functions.
Long chains of amino acids;
Folding of chain into a coil /pleated sheet;
Association of several polypeptide chains together
H bonds / Disulphide bonding (In context);
Fibres provide strength (and flexibility);
Sheets provide flexibility;
Example e.g. keratin in hair, collagen in bone; (MUST be in context)
Insoluble because external R-groups are non-polar
Explain why initial rate of this reaction was faster at 65 °C than it was at 55 °C (3 marks)
Particles have more KE at higher temperatures; move faster
Greater chance of collision
More enzyme-substrate complexes form
Describe the test for starch.
Add iodine solution to the sample.
If the solution goes from orange-brown to blue-black starch is present.
Describe the test for lipids.
Emulsion test - add ethanol to the sample and shake the test tube then add water to it.
Lipids are present if a white emulsion appears.
Describe the test for reducing sugars.
Add Benedict's reagent to the sample then heat the solution gently.
If it changes from light blue to a brick-red precipitate, indicates that reducing sugars are present.
Describe the test for non-reducing sugars.
Add HCl to the sample and heat gently, neutralise the sample with NaHCO3 solution.
Add Benedict's reagent to the sample and heat in water bath
If change from light blue to brick-red precipitate, then result is positive.
Describe the test for proteins.
Add Biuret solution
If proteins present, solution turns from blue to purple
When the reaction with catalase is carried out in a test tube, the test tube feels warm at the end of the reaction. Explain why. (2 marks)
Energy in products less than energy in substrate
Energy is released as heat
A student carried out the Benedict’s test. Suggest a method, other than using a colorimeter, that this student could use to measure the quantity of reducing sugar in a solution. (2 marks)
Filter and dry the sample
Find the mass
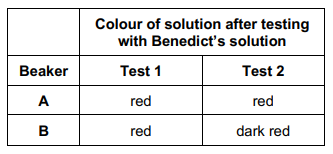
In an investigation, a student wanted to identify the solutions in two beakers, A and B. She knew one beaker contained maltose solution and the other beaker contained glucose solution. Both solutions had the same concentration.
She did two separate biochemical tests on a sample from each beaker.
Test 1 - used Benedict's solution to test for reducing sugar.
Test 2 - added the enzyme maltase, heated the mixture at 30 °C for 5 minutes, and then used Benedict's solution to test for reducing sugar.
Explain the results for beakers A and B in the table. (2 marks)
A = glucose and B = maltose
Because more sugar after hydrolysis
Use of a colorimeter in this investigation would improve the repeatability of the student’s results. Give one reason why. (1 mark)
Gives quantitative results
So standardises the method
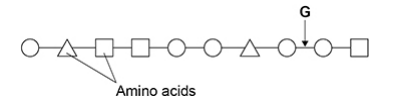
Name the type of peptidase which will hydrolyse the bond labelled G in the diagram. (1 mark)
Endo(peptidase)
Describe the induced-fit model of enzyme action and how an enzyme acts as a catalyst. (3 marks)
Substrate binds to the active site
Active site changes shape (slightly) so it is complementary to substrate
Reduces activation energy
When bread becomes stale, the structure of some of the starch is changed. This changed starch is called retrograded starch. Scientists have suggested retrograded starch is a competitive inhibitor of amylase in the small intestine.
Assuming the scientists are correct, suggest how eating stale bread could help to reduce weight gain. (3 marks)
Less hydrolysis of starch
To maltose
So less absorption of glucose
Describe how the structure of a protein depends on the amino acids it contains. (5 marks)
Structure is determined by (relative) position of amino acids
Primary structure is sequence/order of amino acids
Secondary structure formed by hydrogen bonding
Tertiary structure formed by interactions (between R groups)
Creates active site in enzymes
Explain how the active site of an enzyme causes a high rate of reaction. (3 marks)
Lowers activation energy
Induced fit causes active site (of enzyme) to change shape
So enzyme-substrate complex causes bonds to form/break
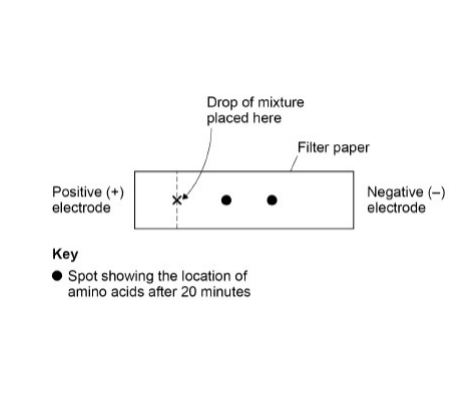
A solution contained a mixture of three different amino acids. A scientist passed an electric current through the solution to separate the amino acids. She placed a drop of the mixture at one end of a piece of filter paper, attached an electrode to each end of the paper and switched on the current, then off the current after 20 minutes and stained the paper to show spots of the amino acids at new positions.
Her results are shown in the diagram.
Explain what the positions of the spots in the diagram show about these amino acids. (3 marks)
Moved to negative electrode because positively charged
Spots move different distances because (amino acids) different charge/mass
Two spots (not three) because these two amino acids same charge/mass
The secondary structure of a polypeptide is produced by bonds between amino acids. Describe how. (2 marks)
Hydrogen bonds
Between NH (group of one amino acid) and C=O
(OR Forming β pleated sheets / α helix)
Formation of an enzyme-substrate complex increases the rate of reaction. Explain how. (2 marks)
Reduces activation energy
Due to bending bonds
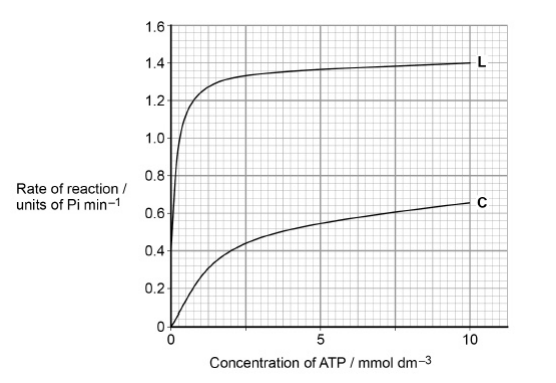
Lyxose binds to the enzyme.
Suggest a reason for the difference in the results shown in the graph with (L) and without lyxose (C). (3 marks)
Binding alters the tertiary structure of the enzyme
This causes active site to change shape
So more successful E-S complexes form
A change from Glu to Lys at amino acid 300 had no effect on the rate of reaction catalysed by the enzyme. The same change at amino acid 279 significantly reduced the rate of reaction catalysed by the enzyme.
Suggest reasons for the differences between the effects of these two changes. (2 marks)
Change at amino acid 300 does not change the shape of the active site
Amino acid 279 may have been involved in a (ionic, disulfide or hydrogen) bond and so the shape of the active site changes
Suggest two variables the biochemist controlled when investigating the effect of temperature on the rate of breakdown of a protein by an enzyme. (2 marks)
Enzyme concentration
pH
Use your knowledge of protein structure to explain why enzymes are specific and may be affected by non-competitive inhibitors. (6 marks)
Each enzyme has specific primary structure / amino acid sequence
Folds in a particular way / has particular tertiary structure giving an active site with a unique structure
Shape of active site complementary to substrate
Inhibitor fits at site on the enzyme other than active site
Distorts active site
So substrate will no longer fit to make enzyme-substrate complex
Describe how a phosphodiester bond is formed between two nucleotides within a DNA molecule. (2 marks)
Condensation reaction
Between phosphate and deoxyribose
Catalysed by DNA polymerase
Describe two similarities and two differences between the structure of a DNA molecule and the structure of a molecule of ATP. (4 marks)
Similarities:
Both contain adenine
Both contain a phosphate group
Differences:
ATP has 3 phosphates, DNA nucleotide has 1
ATP contains ribose, DNA contains deoxyribose
Describe the structure of DNA. (5 marks)
Polymer of nucleotides
Each nucleotide formed from deoxyribose, a phosphate and a nitrogenous base
Phosphodiester bonds between nucleotides
Double helix held by hydrogen bonds
Hydrogen bonds between adenine, thymine and cytosine, guanine
Use your knowledge of semi-conservative replication of DNA to suggest:
1) the role of the single-stranded DNA fragments
2) the role of the DNA nucleotides (3 marks)
Role of single-stranded DNA fragments:
Template
Determines order of nucleotides/bases
Role of DNA nucleotides:
Forms complementary pairs (A – T, G - C)
Describe the role of two named enzymes in the process of semiconservative replication of DNA. (3 marks)
DNA helicase causes breaking of hydrogen bonds between DNA strands
DNA polymerase joins the DNA nucleotides
Forming phosphodiester bonds
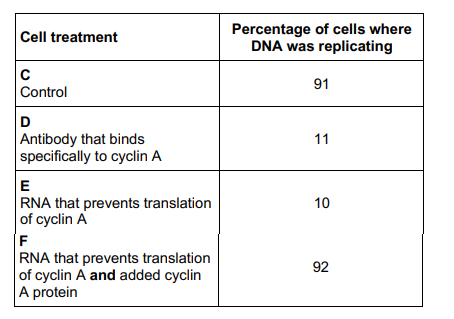
Scientists investigated the function of a eukaryotic cell protein called cyclin A which is thought to be involved with the binding of one of the enzymes required at the start of DNA replication.
Treated cultures of cells in the following ways:
C – Control cells, untreated
D – Added antibody that binds specifically to cyclin A
E – Added RNA that prevents translation of cyclin A
F – Added RNA that prevents translation of cyclin A and added cyclin A protein
They then determined the percentage of cells in each culture in which DNA was replicating. Their results are shown in the table.
Suggest explanations for the results in the table. (3 marks)
Treatment D - Antibody binds to cyclin A so it cannot bind to DNA and initiate DNA replication
Treatment E - RNA interferes with polypeptide formation (so cyclin A not made)
In Treatment F added cyclin A can bind to enzyme to initiate DNA replication
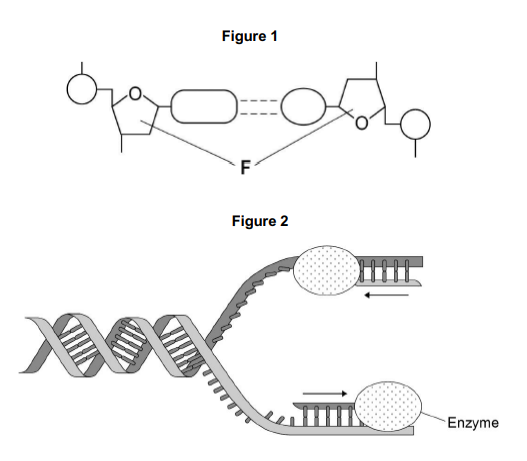
The arrows in Figure 2 show the directions in which each new DNA strand is being produced.
Use Figure 1, Figure 2 and your knowledge of enzyme action to explain why the arrows point in opposite directions.
Figure 1 shows that DNA has antiparallel strands
Also shows shape of the nucleotides is different
Enzymes have active sites with specific shape
Only substrates with complementary shape can bind with active site of enzyme
Give two ways in which the hydrolysis of ATP is used in cells. (2 marks)
To provide energy for other reactions
To add phosphate to other substances and make them more reactive/change their shape;
ATP is useful in many biological processes. Explain why. (4 marks)
Releases energy in small, manageable amounts
Broken down in one step
Immediate energy compound
Can be made again
Describe how an ATP molecule is formed from its component molecules. (4 marks)
Adenine, ribose, three phosphates
Joined in a condensation reaction
Catalysed by ATP synthase
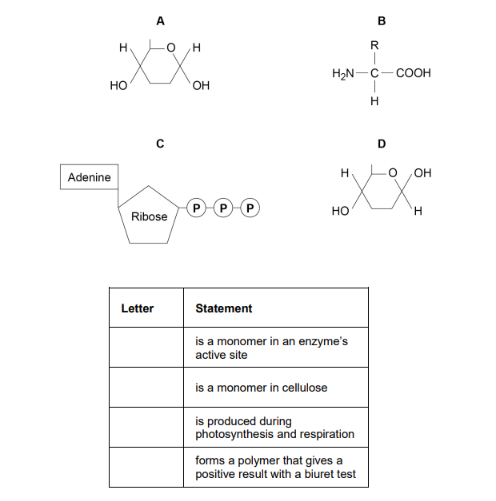
The diagram shows the structure of molecules found in organisms
Complete the table by putting the correct letter, A, B, C or D, in the box next to each statement. Each letter may be used once, more than once, or not at all. (4 marks)
B
D
C
B
State and explain the property of water that can help buffer changes in temperature. (2 marks)
Water has a relatively high specific heat capacity
Can gain / lose a lot of heat without changing temperature
Explain five properties that make water important for organisms. (5 marks)
A metabolite in photosynthesis/respiration
A solvent so allowing transport of substances
High specific heat capacity so buffers changes in temperature
Large latent heat of vaporisation so provides a cooling effect through evaporation
Cohesion between water molecules so supports columns of water (in plants)
Give two properties of water that are important in the cytoplasm of cells. For each property of water, explain its importance in the cytoplasm.
Universal solvent
Metabolic reactions occur faster in solution
Reactive
Takes place in hydrolysis / condensation
A high concentration of sodium in the blood can affect blood volume and cause hypertension.
Use your knowledge of water potential to suggest how high sodium concentrations in the medicines taken could affect blood volume. (3 marks)
Sodium ions lower the water potential of blood
Water would move into the blood by osmosis
Increasing the blood volume
Describe the roles of iron ions, sodium ions, and phosphate ions in cells. (5 marks)
Iron ions:
Haemoglobin binds/associates with oxygen
Sodium ions:
Co-transport of glucose/amino acids (into cells)
Affects osmosis/water potential
Phosphate ions:
Phosphorylates other compounds making them more reactive
Hydrophilic part of phospholipid bilayer
During replication, the two DNA strands separate and each acts as a template for the production of a new strand. As new DNA strands are produced, nucleotides can only be added in the 5’ to 3’ direction. Use the figure in part (a) and your knowledge of enzyme action and DNA replication to explain why new nucleotides can only be added in a 5’ to 3’ direction.
(Reference to) DNA polymerase
Which is specific
Only complementary with binds to 5’ end of strand
Shapes of 5’ end and 3’ end are different