[ 2FINAL ] Chemistry - Stoichiometry
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
35 Terms
Stoichiometry
A branch of chemistry that deals the quantitative relationships between reactants and products in a chemical reaction.
It allows chemistry to calculate how much of each substance is needed or produced in a reaction based on a balanced chemical equations.
Methane
What does this equation CH4 represent?
Ethane
What does this equation C2H6 represent?
Propane
What does this equation C3H8 represent?
Butane
What does this equation C4H10 represent?
Hexane
What does this equation C6H14 represent?
Pentane
What does this equation C5H12 represent?
Heptane
What does this equation C7H16 represent?
Atom
What kind of particle are these?
Na, Ca, Li
Ion
What kind of particle are these?
Na+ , Ca2+ , Li+
Molecule
What kind of particle are these?
H2 , H2O , CO2
Formula Unit
What kind of particle are these?
NaCl , LiF , KI
Molar Mass
the mass in grams of one mole of a substance
expressed in grams/mole or g/mol
calculated by summing the atomic masses of all the atoms in a molecule/ formula unit
Combustion
The process of burning a substance (usually fuel) in the presence of oxygen to produce CO2 AND H2O, releasing energy in the form of heat and light.
Complete Combustion
This happens when there is a good or sufficient supply of air or oxygen.
Produces a blue flame
Produces water vapor and carbon dioxide
Blue
What color flame does a complete combustion produce? This indicates that the gas is being burned efficiently without any unburned and wasted gas.
Incomplete Combustion
This happens when the supply of air or oxygen is poor or insufficient.
less energy is released than during complete combustion
Produces a yellow flame
Water, carbon monoxide, and carbon are produced
Yellow
What color flame does an incomplete combustion produce?
Flame
1
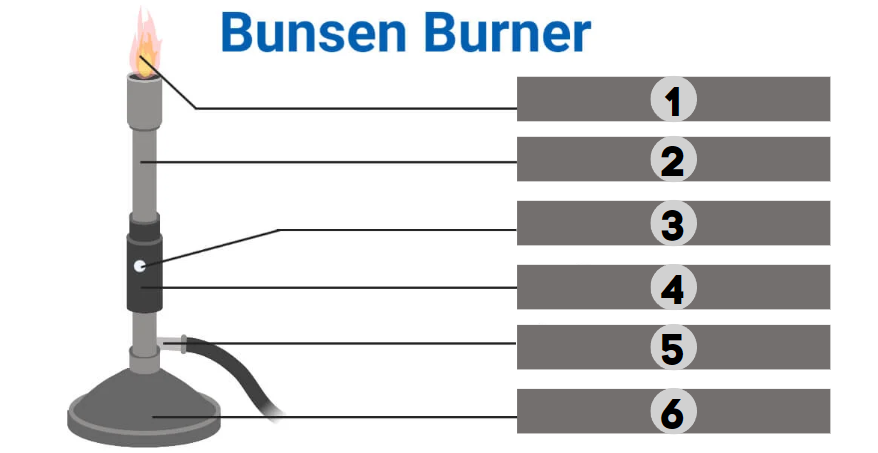
Barrel
2
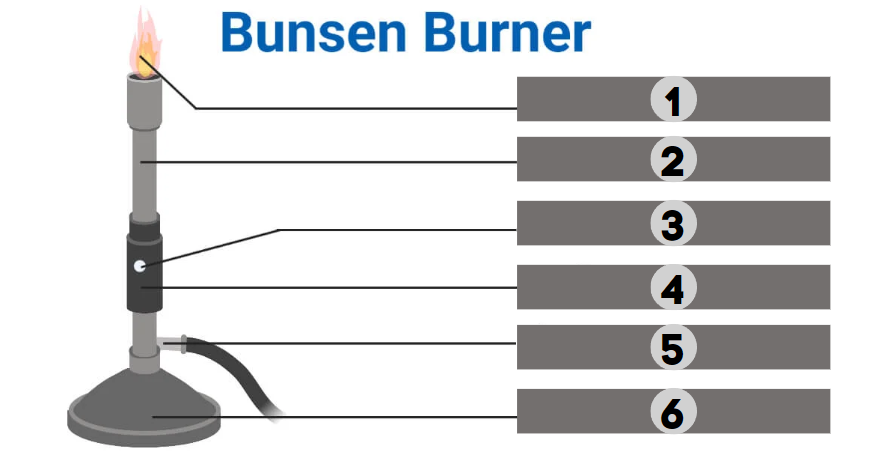
Air Hole
3
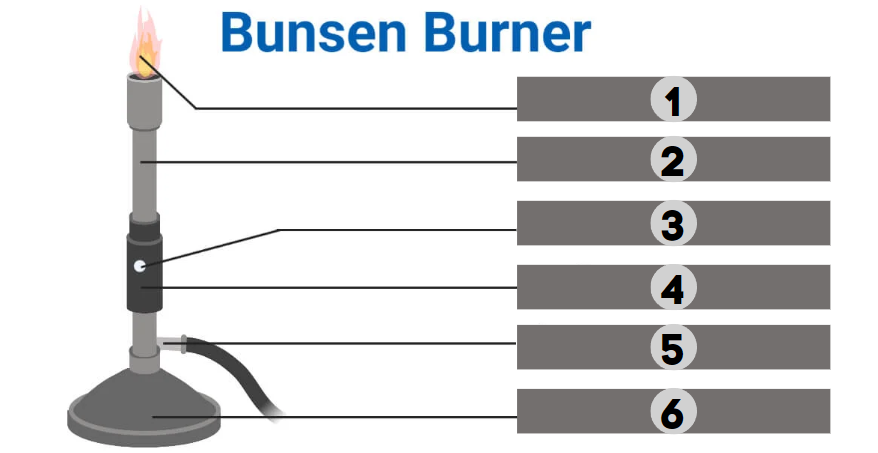
Collar
4
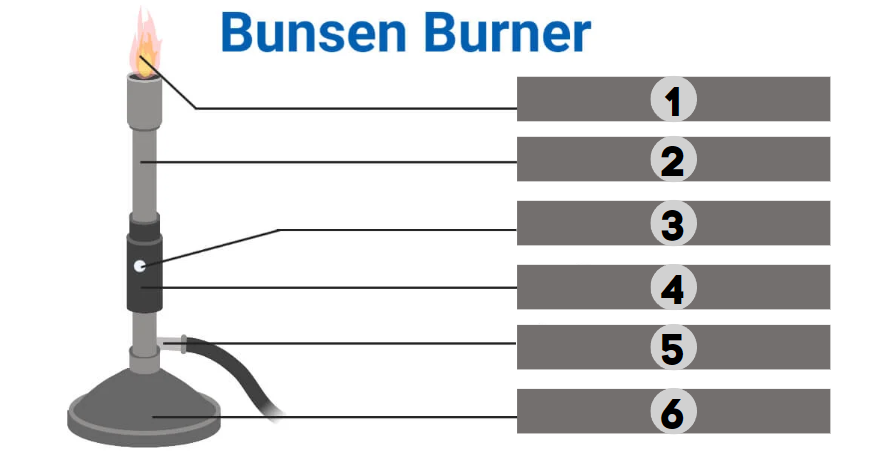
Gas
5
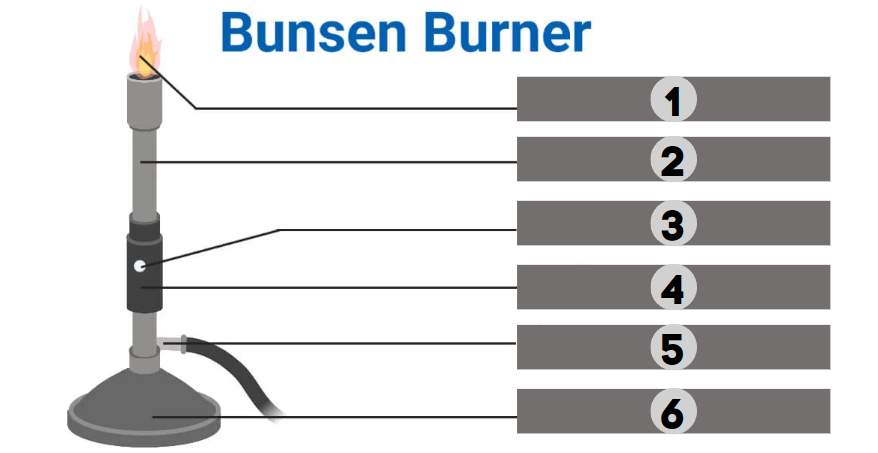
Base
6
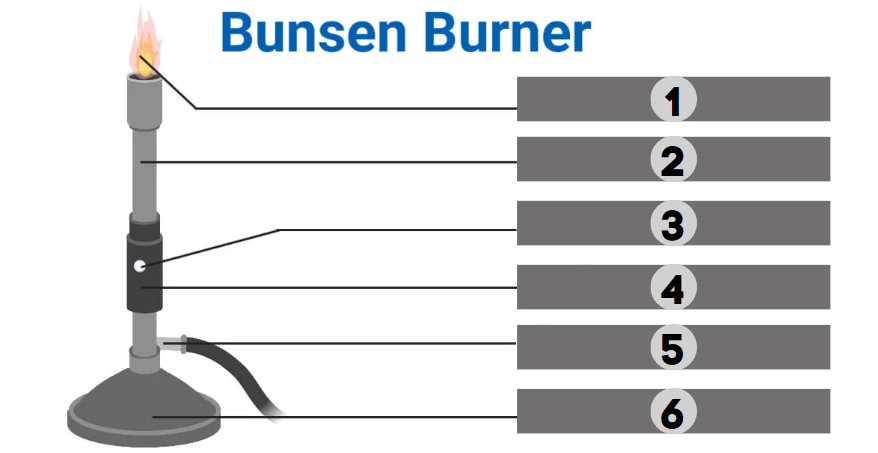
Base
The bunsen burner is supported by this, a broad and hefty component that comes in various shapes. It’s attached to a side tube known as a gas tube. It helps to place the burner on the bench.
Barrel
It is a base-attached vertical metal tube about 5 inches long. It has an air vent near the bottom formed by oppositely positioned holes. This can be fastened to the base with screws.
Collar
It joins the base and the barrel. It is a small, cylindrical piece of metal with two holes that are opposite of one another. It regulates how much air enters the barrel.
Air hole
This allows air to enter the burner to form a mixture of air and gas or any liquid fuel with air.
Red
What is the color of the flame when it reacts with LiCl?
Green
What is the color of the flame when it reacts with BaCl2?
Lilac/Purple
What is the color of the flame when it reacts with KI+?
Orange
What is the color of the flame when it reacts with CaCl?
Electron Excitation
This is the transfer of a bound electron to a more energetic, but still bound state.
Closest to Nucleus
Where do electrons have the lowest energy?
Flame Test
This involves introducing a sample of the element or compound to a hot, non-luminous flame and observing the color of the flame that results.