Chapter 2, Chemical foundations: Covalent Bonds and Noncovalent Interactions, Chemical Building Blocksof Cells
1/143
Earn XP
Description and Tags
Bolded vocab words from slides defined by textbook glossary
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
144 Terms
Molecular Complementarity
Lock-and-key kind of fit between the shapes, charges, hydrophobicity, and/or other physical properties of two molecules or portions thereof that allow formation of multiple noncovalent interactions between them at close range.
Allows molecules with complementary shapes and chemical properties to form tight complexes.
Drives Specificity and Affinity.
One of the four key concepts for the chemistry of life.
Chemical Building Blocks
Small molecules join to form larger cellular structures and polymers like DNA and proteins- the macromolecules.
One of the four key concepts for the chemistry of life.
Chemical equilibrium
The state of a chemical reaction in which the concentration of all products and reactants is constant because the rates of the forward and reverse reactions are equal.
Ratio of products and reactants depends on the rate constants (k) of the forward and reverse reactions. Keq provides a measure of their amounts at equilibrium.
Steady State is at a chemical equilibrium bc equilibrium = death.
One of the four key concepts for the chemistry of life.
Chemical Bond Energy
Energy driving many cellular activities is derived from hydrolysis of high-energy bonds, like the phosphoanhydride bonds in Adenosine Triphosphate (ATP).
One of the four key concepts for the chemistry of life.
Covalent Bond
Stable chemical force that holds the atoms in molecules together by sharing of one or more pairs of electrons.
The strongest of the BIOLOGICAL atomic interactions, key to establishing the structure of organic molecules.
Ionic bonds aren’t the strongest bc biological environment happens in water and ions are dissolved in water.
Molecules
Stable combinations of atoms held together by covalent bonds
Noncovalent interaction
Any relatively weak chemical interaction that does not involve an intimate sharing of ekectrons.
Ionic bonds, Hydrogen bonds, van Der Waal’s interactions and the hydrophobic effect, weak but are still instrumental for both shape and chemistry of biological molecules.
Important for dynamic cellular processes.
Most Abundant elements in biological molecules.
Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, and Sulfur.
CHNOPS
Carbon’s presence defines organic molecules.
Stereoisomers
Two compounds with identical molecular formulas whose atoms are linked in the same order but in different spatial arrangements. In optical isomers, designated D and L, the atoms bonded to an asymmetric carbon atom are arranged in a mirror-image fashion. Geometric isomers include the cis and trans forms of molecules containing a double bond.
Same molecular formula, same connectivity, but different arrangement of atoms around chiral atom.
Nonpolar
Referring to a molecule or structure that lacks any net electric charge or asymmetric distribution of positive and negative charges. Nonpolar molecules generally are less soluble in water than polar molecules and are often water insoluble.
electrons spend equal amount of time around both nulei in the bond.
Polar
Referring to a molecule or structure with a net electric charge or asymmetric distribution of positive and negative charges. Polar molecules are usually soluble in water.
Electrons pulled toward one of the nuclei, generates weak positive and negative charge on two atoms.
Electronegativity
The extent of an atom’s ability to attract an electron. in CHNOPS oxygen is the most electronegative. trends towards F on periodic table.
Dipole
A positive charge separated in space from an equal but opposite negative charge.
Dipole Moment
A quantitative measure of the extent of charge separation, or strength, of a dipole, which for a chemical bond is the product of the partial charge on each atom and the distance between the two atoms.
Functional Groups
Covalently attached molecular units confer distinct chemical properties to resulting molecules.
Alcohol
-OH
hydroxyl
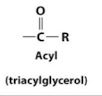
Triacylglycerol
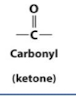
Ketone
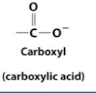
Carboxylic Acid
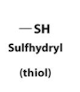
Thiol
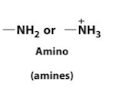
Amines
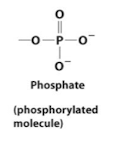
Phosphorylated molecule
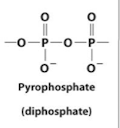
diphosphate
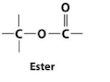
Ester
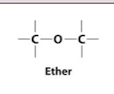
Ether
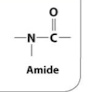
Amide
Linkage
A small number of covalent bonds types are foundational to the formation of organic molecules.
Ionic bond (ionic interaction)
A noncovalent interaction between a positively charged ion (cation) and negatively charged ion (anion)
Weakened in the presence of water, a hydration shell, other ions in solution.
Cation
A positively charged ion
Anion
a negatively charged ion
Hydrogen Bond
A noncovalent interaction between an atom (commonly oxygen or nitrogen) carrying a partial negative charge and a hydrogen atom carrying a partial positive charge. Important in stabilizing the conformation of proteins and in formation of base pairs between nucleic acid strands.
Promote: protein structure, annealing of DNA strands, Interactions between molecules.
Van Der Waals Interaction
A weak noncovalent interaction due to small, transient asymmetric electron distributions around atoms (dipoles).
Hydrophobic Effect
The tendency of nonpolar molecules or parts of molecules to associate with each other in aqueous solution so as to minimize their direct interactions with water; commonly called a hydrophobic interaction or bond.
Complementary
(1) Referring to two nucleic acid sequences or strands that can form perfect base pairs with each other. (2) Describing regions on two interacting molecules (e.g., an enzyme and its substrate that fit together in a lock-and-key fashion.
Specificity
The ability of immune cells or their products to distinguish between structurally closely related molecules.
Interactions between two molecules, for example an antibody to an antigen.
Affinity
Tightness of association. Greater complementarity and number of non-covalent interactions produce a tighter association between two molecules.
Dehydration Reaction
The net loss of a -H from one monomer and a hydroxyl (-OH) from the other resulting in a covalent bond linkage.
Hydrolysis
Breaking bonds by the addition of a water molecule equivalent an -H and -OH.
Peptide Bond
The covalent amide linkage between amino acids formed between the amino group of one amino acid and the carboxyl group of another with the net release of a water molecule (dehydration).
Phosphodiester Bond
(ester) Chemical linkage between adjacent nucleotides in DNA and RNA; consists of two phosphoester bonds, one on the 5’ side of the phosphate and another on the 3’ side.
Glycosidic Bond
The covalent linkage between (ethers) two monosaccharide residues formed when a carbon atom in one sugar reacts with a hydroxyl group on a second sugar with the net release of a water molecule (dehydration).
Fats
Glycerol (3-carbon sugar) linked by ester bonds to three fatty acids.
Fatty Acid
Any long hydrocarbon chain that has a carboxyl group at one end; a major source of energy during metabolism and a precursor for synthesis of phospholipids, triglycerides, and cholesteryl esters.
Amphipathic (or amphiphilic)
Referring to a molecule or structure that has both a hydrophobic and a hydrophilic part.
Amino Acid
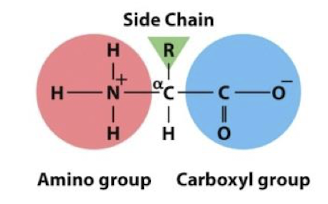
Peptide Bond
Amino Acids are linked by covalent bonds from dehydration between amino group and carboxylic acid of another (forming an amide group).
Polypeptide
Linear polymer of amino acids connected by peptide bonds, usually containing 20 or more residues.
Polymers of amino acids
Residue
General term for the repeating units in a polymer that remain after covalent linkage of the monomeric precursors.
How amino acid is referred to when part of a polypeptide.
Aspartate
Hydrophilic
Charged
Acidic
COO-
Asp
D
Glutamate
Hydrophilic
Charged
Acidic
COO-
Glu
E
Lysine
Hydrophilic
Charged
Basic
NH3+
Lys
K
Arginine
Hydrophilic
Charged
Basic
=NH2+
Arg
R
Histidine
Hydrophilic
Charged
not ionized at pH 7 has a pka ~6 so can act both basic and acidic based on pH and interactions
His
H
~5.8 N+ at 7.8 N neutral
Hydrophilic Charged
Can form ionic bonds
Asparagine
Hydrophilic
Polar uncharged
Amide group
Asn
N
Glutamine
Hydrophilic
Polar uncharged
Amide group
Gln
Q
Serine
Hydrophilic
Polar uncharged
Hydroxyl group
Ser
S
Threonine
Hydrophilic
Polar uncharged
Hydroxyl group
Thr
T
Hydrophilic uncharged
Can form hydrogen bonds because of their polarity.
Alanine
Nonpolar
Ala
A
Valine
Nonpolar
Val
V
Isoleucine
Nonpolar
Ile
I
Leucine
Nonpolar
Leu
L
Methionine
Nonpolar
Met
M
Phenylalanine
Nonpolar
Phe
F
Tyrosine
Nonpolar
Tyr
Y
Tryptophan
Nonpolar
Trp
W
Glycine
Special amino acid
side chain is an -H therefore not chiral, makes backbone very flexible and very useful for protein hinges.
Cysteine
Special Amino Acid
side chain is a -CH2-SH which form disulfide bridge with other cysteines that reinforce and maintain protein conformation.
Proline
Special Amino Acid
side chain forms a covalent bond with the amino group generating ring. Bent shape creates kink in the polypeptide.
Nonpolar
Cannot interact with water to form electrostatic bonds. Generally associate with one another, and are repelled to the protein interior by the aqueous surroundings.
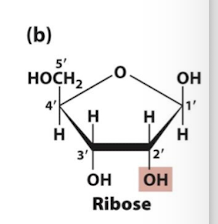
Ribose
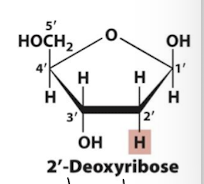
exposed -OH is a target for biochemical reactions, so RNA is less stable than DNA because of this
2’-Deoxyribose
Purines
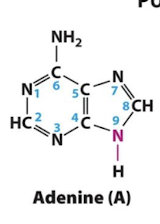
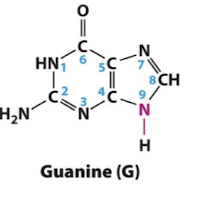
Adenine, Guanine
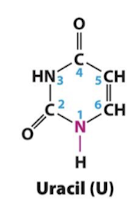
Pyrimidines
Uracil, Thymine, Cytosine
Ademine
Guanine
Uracil
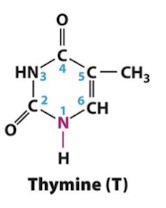
Thymine
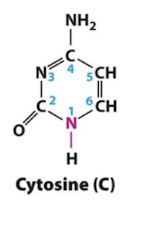
Cytosine
Nucleotide
A nucleoside with one or more phosphate groups linked via an ester bond to the sugar moiety, generally to the 5’ carbon atom. DNA and RNA are polymers of nucleotides containing deoxyribose and ribose, respectively.
Nucleoside
A small molecule composed of a purine or pyrimidine base linked to a pentose (either ribose or deoxyribose).
Purines
A class of nitrogenous compounds containing two fused heterocyclic rings. Two purines, adenine (A) and guanine (G), are base components of nucleotides found in DNA and RNA.
Pyrimidines
A class of nitrogenous compounds containing one heterocyclic ring. Two pyrimidines, cytosine (C) and thymine (T), are base components of nucleotides found in DNA; in RNA, uracil (U) replaces thymine.
Ketones
Carbonyl on an interanal carbon (ketose)
Aldehydes
Carbonyl on a terminal carbon (aldose)
Glycosidic Bonds
Result from dehydration reactions between the -OH of the chiral carbon and -OH of a carbon in another sugar
-c-o-c- link between sugars
Disaccharide
A small carbohydrate (sugar) composed of two monosaccharides covalently joined by a glycosidic bond.
readily avaliable energy
Oligosaccharides
3-10 sugars bind to cell surface proteins and lipids, used in cell recognition, alter protein solubility and ability to interact with other proteins.
Polysaccharide
Linear or branched polymer of monosaccharides linked by glycosidic bonds and usually containing more than 15 residues. Those with fewer than 15 residues are often called oligosaccharides.
storage
Alpha diastereomer
-OH is below the plane of the ring
storage
Beta
-OH is above the plane of the ring
structural
digestive system can’t break beta 1-4 linkages
Glycogen
A very long, branched polysaccharide, composed exclusively of glucose units, that is the primary storage carbohydrate in animals; found primarily in liver and muscle cells.
Starch
A very long, branched polysaccharide, composed exclusively of glucose units, that is the primary storage carbohydrate in plant cells.
Triacylglycerol (triacylglyceride)
Major form in which fatty acids are stored and transported in animals; consists of three fatty acyl chains esterified to a glycerol molecule.
Saturated
Referring to a compound (e.g., fatty acid) in which all the carbon-carbon bonds are single bonds.
Unsaturated
Referring to a compound (e.g., fatty acid) in which one of the carbon-carbon bonds is a double or triple bond.
Polyunsaturated
Referring to a compound (e.g., fatty acid) in which two or more of the carbon-carbon bonds are double or triple bonds.
Steroids
Derived from cholesterol
Hydrocarbon skeleton with 4 rings
Different functional groups attached to each steroid
Mostly hydrophobic
Phosphoglycerides
Amphipathic derivatives of glycerol 3-phophate that generally consist of two hydrophobic fatty acyl chains esterified to the hydroxyl groups in glycerol and a polar head group attached to the phosphate; the most abundant lipids in biomembranes.
Major component of the cell membrane
glycerol back bone
2 fatty acids
phosphate group with small polar group attached