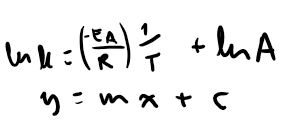Arrhenius equations
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Write down the arrhenius equation when k is the subject of the equation

Write down the arrhenius equation when ln k is the subject of the equation

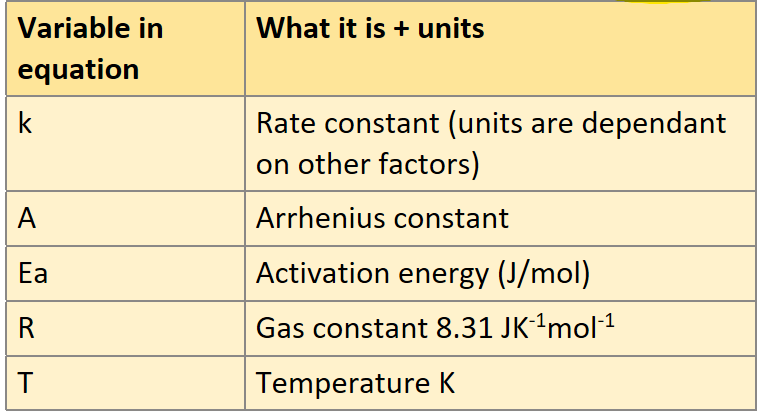
Write down all the variables in the equation and their units
Why does activation energy get smaller as rate constant gets bigger?
As activation energy drops, rate of reaction increases. Many more particles have enough energy to react when they collide.
Why does rate constant increase as temperature increases?
When temperature increases, the particles have more kinetic energy and are more likely to collide with each other with at least the activation energy. So rate of reaction increases.
Write down the arrhenius equation in terms of y= mx +c