Pharmacology of Type II Diabetic Drugs
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
34 Terms
What is diabetes and what is its main effect?
- A chronic disease wherein the pancreas does not produce enough insulin or the body cannot effectively use it
- Hyperglycaemia, the main effect of uncontrolled diabetes, can damage various systems esp nerve and blood vessels
What are the 4 different types of Diabetes
1. Diabetes Mellitus Type I
2. Diabetes Mellitus Type 2
Less common:
3. Gestational Diabetes - occurs during pregnancy
4. Diabetes Insipidus - diabetes due to other causes
What is type I?
Autoimmune destruction upon Beta-cells causing insulin deficiency
Dependent on insulin
What is type II?
Type II [Non-insulin dependent diabetes]:
Insulin resistance, causing target tissue to be unresponsive and have impaired insulin secretion
Later onset associated with diet and lifestyle e.g., obesity and milder
Major incidence increase including children
Hyperglycaemia but usually no ketoacidosis
Often responds to diet and oral hypoglycaemic agents
How does GLP-1 work?
When food is ingested outline the 2 pathways it triggers?
1ST PATHWAY: GLP-1 SECRETION
After the consumption of food and digestion is initiated, glucose levels start to increase and
Hormones such as GLP-1 are released in the intestines
GLP - 1 is an incretin → a hormone that helps regulate insulin and glucose levels in the body
Triggers insulin (insulin acts to decrease glucose levels)
Inhibits glucagon
Induces feeling of satiety
Reduce appetite
2nd pathway: Pancreatic hormones SECRETION
- Food consumption also triggers release of pancreatic hormones like insulin, amylin and glucagon
- Insulin and amylin work to decrease glucose levels and inhibit glucagon while glucagon acts on the liver to raise glucose levels
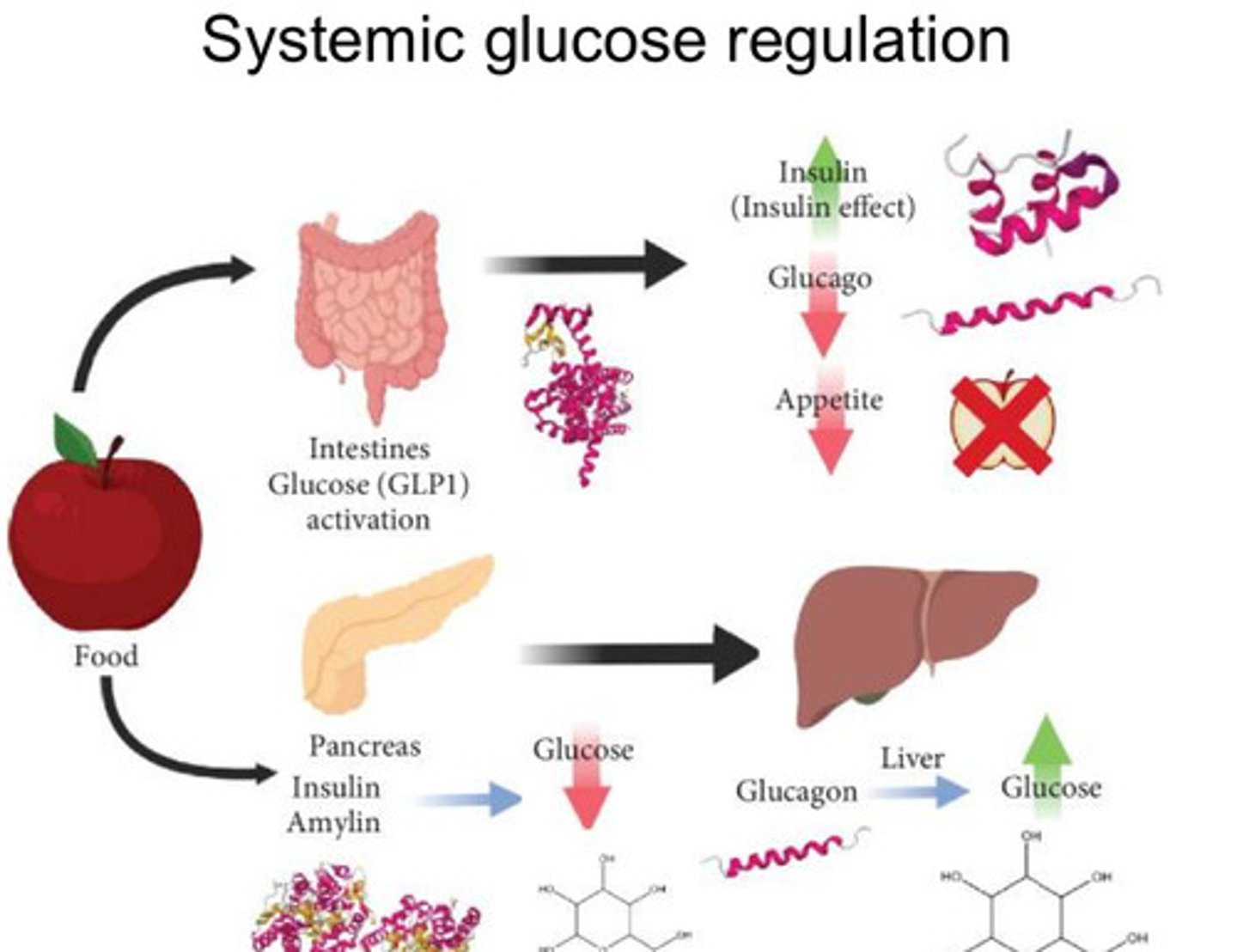
How does glucagon work?
Acts on liver to raise glucose
Outline the goal of Diabetes management, diet suggestions and main treatments for Type I and Type II Diabetes
- Goal of management: keep blood glucose levels as close to normal as safely possible
- Diet: decrease carbohydrate intake
- Type I: insulin (IDDM)
- Type II: Biguanides, Incretins, DPP4, SGLT2I, amylin analogues, insulin
What is the main agent for type I?
Insulin injection
What are the main agents for type II?
Biguanides
Incretins
DPP4-I
SGLT2-I
Amylin analogues
What is the main biguanide? How does it work?
- Metformin
- Absorbed from small intestine - half-life is 3 hours, unbound to plasma protein and excreted unchanged in the urine
It decreased blood glucose concentration by:
- inhibit genes involved in gluconeogenesis in liver
- enhances insulin action on muscle and adipose
- stimulate glycolysis and uptake in tissue
- Decreases carbohydrate absorption
Generally as drug combinations: given with incretins, DPP4 inhibitors, SGLT2 inhibitors, sulphonylureas, thiazolidinediones and/or insulin
Why is Metformin preferred?
It does NOT cause:
- hypoglycemia
- stimulated appetite
- Cause weight gain
- It reduces microvascular complications
What are Metformin SEs?
Diarrhoea
Nausea
Metallic taste
Reduced folate and B12 absorption (may need to take supplements)
What is a complication of metformin?
Lactic acidosis (rare)
Which anti-type II drugs cause hypoglycaemia?
Sulphonylureas
Thioazolidiones
Insulin
What is the mechanism of action of Metformin?
- Does NOT directly affect insulin, glucagon, GH, cortisol, somatostatin
- Activates AMP-activated protein kinase (AMPK) which inhibits anabolic gluconeogenesis and promotes catabolic lipid oxidation which prevents insulin resistance
- AMPK activation enhances insulin sensitivity
- Increases intestinal GLP-1 release which enhances glucose induced insulin secretion and glucagon inhibition
- Main way it is thought to act is inhibiting complex-1 in mitochondria which alters ATP-ADP ratio = activation of AMPK
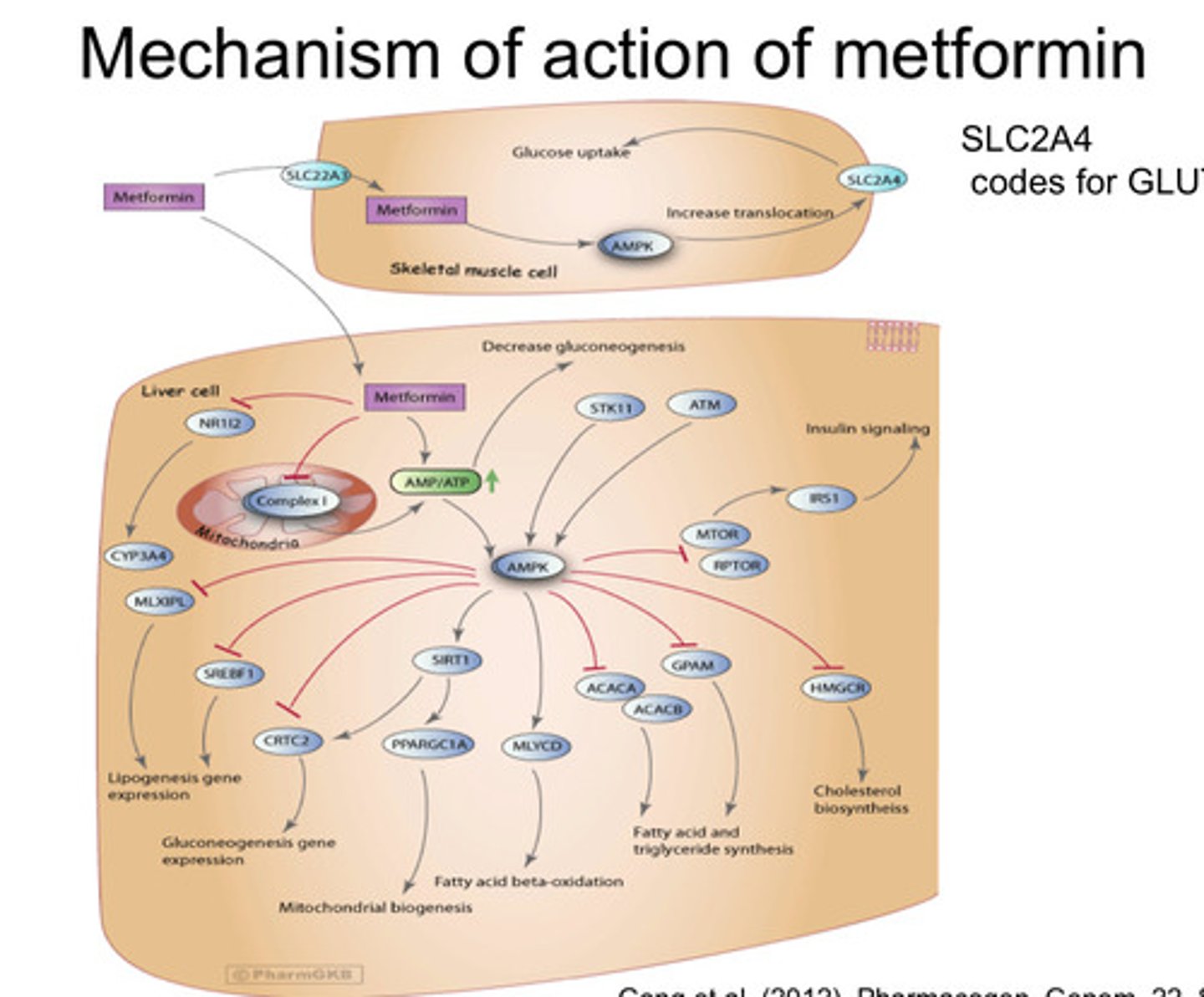
What formulation of insulin causes more insulin release ORAL TABLETS or IV and why?
- Oral glucose causes more insulin release compared to IV due to oral administration going quickly through the gut where secretion of gut factors called incretins are
What are incretins?
- GLP-1: glucagon-like peptide 1 (made by L-cells in distal ileum)
- GIP: gastric inhibitory peptide / glucose-dependent insulinotropic peptide (made by K- cells in duodenum)
- stimulate insulin release and preserve beta cells
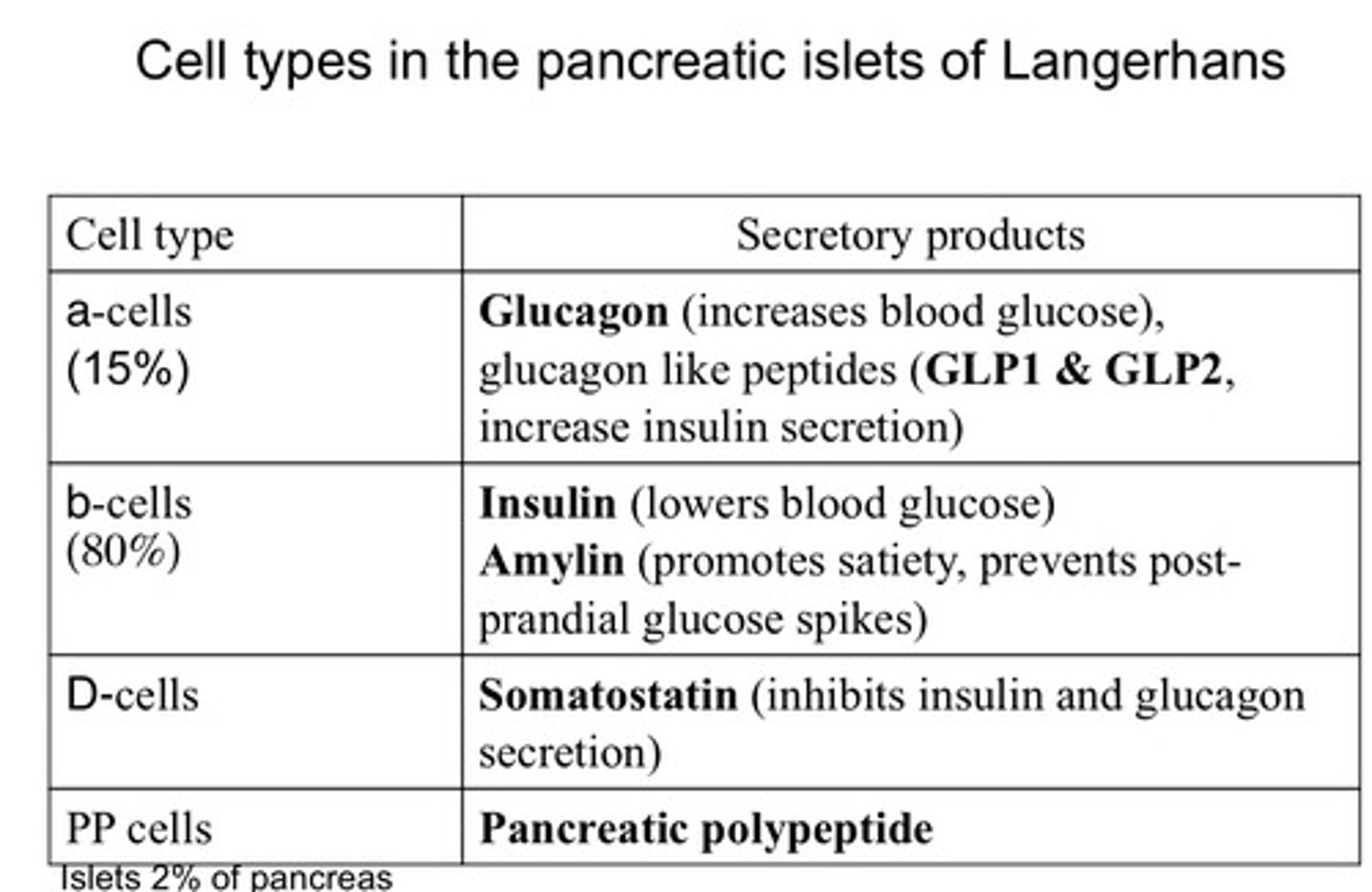
What are the types of pancreatic cells and what do they secrete?
- Islets secrete insulin and amylin
- Alpha cells secrete incretins GLP-1/2 and GIP as well as glucagon
- Delta cells secrete somatostatin which inhibits both insulin and glucagon
- Pancreatic polypeptide cells - secrete pancreatic polypeptide which inhibits insulin
Cell types in islets of Langerhans summary
How are Incretins and Glucagons made in the body?
Glucagons and incretins are made from the same pre-cursor
Posttranslational processing of preproglucagon
The preproglucagon gene encodes proglucagon, a peptide that is differentially processed based on the relative activities of the prohormone convertases 1/3 and 2.
In the α-cells of the pancreatic islet, prohormone convertase 2 (Psck2 - peptidase that cuts the precursor) predominates and glucagon, glicentin-related pancreatic polypeptide (GRPP), intervening peptide 1 (IP1), and a proglucagon fragment are the more prevalent products
—
In the intestinal L-cell and specific CNS neurons, prohormone convertase 1 (Psck1) action is relatively greater and proglucagon is cleaved to GLP-1, GLP-2, oxyntomodulin, glicentin, and IP2.
- Most recent evidence indicates that α-cells have some Prohormone Convertase 1/3 activity, and it is likely that neurons and L-cells also have Prohormone Convertase 2.
What are incretin mimetics (drug), what is a weakness of it and suggest a solution?
Example: Semaglutide = Ozempic / Exendin-4 isolated from saliva of Glia monster and then Exenatide was formulated as the synthetic version
Act on GLP-1 receptor
Stimulate insulin release
Suppress glucagon
Reduce appetite and weight
Slow gastric emptying
Stimulate beta cell proliferation
—
Weakness: It is very unstable - rapidly cleaved by DPP4 is part of the reason for this
Solution: Structural changes of amino acids in GLP-1 analogues cause peptide to be more stable and useful
What are DPP-4 inhibitors?
DPP4 - inhibitors BLOCK the degradation caused by DPP-4 WHICH rapidly degrade incretins within minutes
No effect on weight and no hypoglycaemia
Possible SE: increase in incidence of some cancers
Examples: Sitagliptin and Linagliptin
What are SGLT-2 inhibitors?
- Glucose reabsorbed by SGLT-2 and SGLT-1 in proximal/distal tubules of the Kidney
- Ideally want a drug that is better at inhibiting SGLT-2 than SGLT-1 (as more reabsorbed at SGLT-2 receptor)
- Inhibits SGLT-2 so glucose not reabsorbed
Example: Dapagliflozin and Canagliflozin
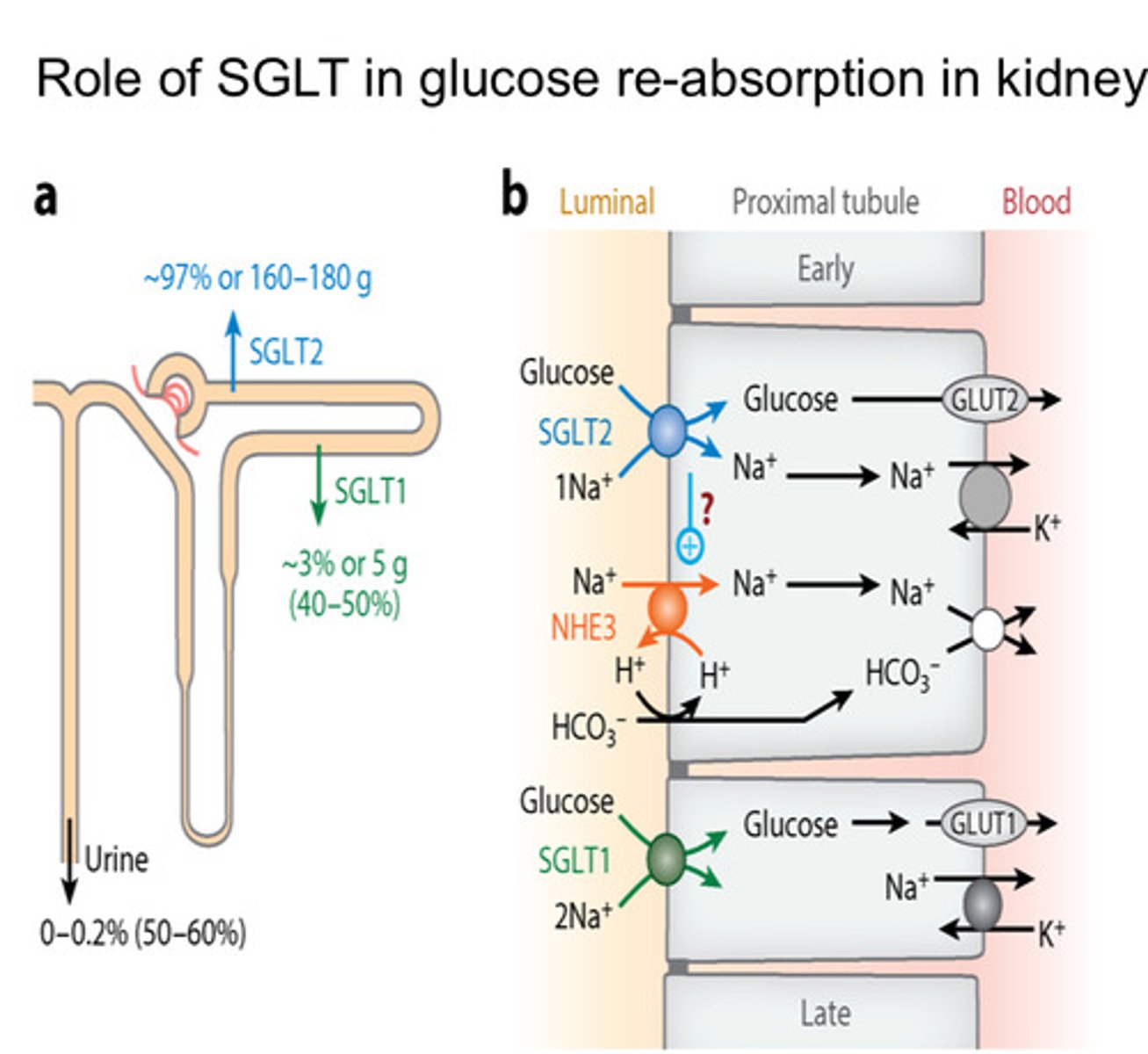
How do sulphonylureas work?
Acutely increase insulin release, increase plasma insulin concentration and decrease hepatic insulin clearance
MOA:
- Inhibitor binds to sulphonylurea receptor and closes ATP-K channel, causing depolarisation of the pancreatic beta cell membrane, causing insulin secretion
- Receptor can be desensitised in chronic use
- Largely protein bound increased interactions with NSAIDs/MAOIs/anitbiotics
-This happens regardless of having high or low glucose
- Excreted in urine with enhanced effect in elderly and renal impairment
- Main adverse effect is hypoglycaemia but also causes neuroglycopenia (lack of glucose supply to top part of brain), confusion/coma -> solution is to take oral glucose
- If severe give: IV glucose, glucagon, adrenaline
Examples:
1st generation: Carbutamide and tolbutamide
2nd generation: Glizlazide, Glimepiride and Gliplizide
What are the effects of chronic exposure of sulphonylureas to patients
Chronic exposure to this drug effects:
Down-regulation of sulphonylurea receptor
Chronic hyperglycaemia as it decreases insulin release
Largely protein bound ~90-99% increases likelihood of drug interactions with NSAIDS, MAOIs, some antibiotics (Rifampicin)
No acute increase in insulin release BUT a decreased plasma glucose concentration still remains
How is the MOA of sulphonylureas and ATP generation similar?
Inhibitors have same effect as ATP generation - bind to a site of sulphonylureas receptor and close ATP-sensitive K channel = depolarises eventually secretes insulin
- When glucose is taken up by the cell it goes into mitochondria where it is oxidised and ATP is generated
- ATP closes an ATP-sensitive K channel which has sulphonylureas as a co- factor
- When ATP closes channel = depolarises beta cell = opens calcium channel = calcium ion influx = stimulates exocytosis of secretory granules = insulin secreted out from beta cells
How do meglitinides work? compare them to sulphonylureas
These drugs have a rapid and less sustained release with a half-life of 1 hour, and are less potent than Sulphonylureas.
Taken just before meal and can be used as mono-therapy.
They function by closing the ATP sensitive K+ channels on beta-cells, with a greater selectivity for the beta-cell than cardiac/vascular ATP sensitive K+ channels.
They share 2 binding sites with SUR but have their own distinct binding site.
Repaglinide and Nateglinide
How do Thiazolidinediones (glitazones) work?
Pioglitazone is only for Type ll DM
A selective agonist for PPAR-gamma, which is found in adipose tissue but also in muscle and liver. The binding of pioglitazone to PPAR-gamma, complexes with the RXR. It recognises the hormones response element.
MOA:
Activates transcriptional genes in glucose homeostasis and lipid metabolism.
Causes changes in lipidgenesis, which has effect on:
Needs insulin to be effective.
Increases glucose uptake in muscle and adipose tissues.
Reduces insulin resistance in peripheral tissues
Pioglitazone has a half-life of approx. 7 hours and takes 6-12 weeks for max effect to develop.
It is given with metformin, insulin or other hypoglycaemic drugs.
Adverse effects include weight gain (1-4kg) due to differentiation of adipocytes and fluid retention by stimulating amiloride sensitive sodium absorption.
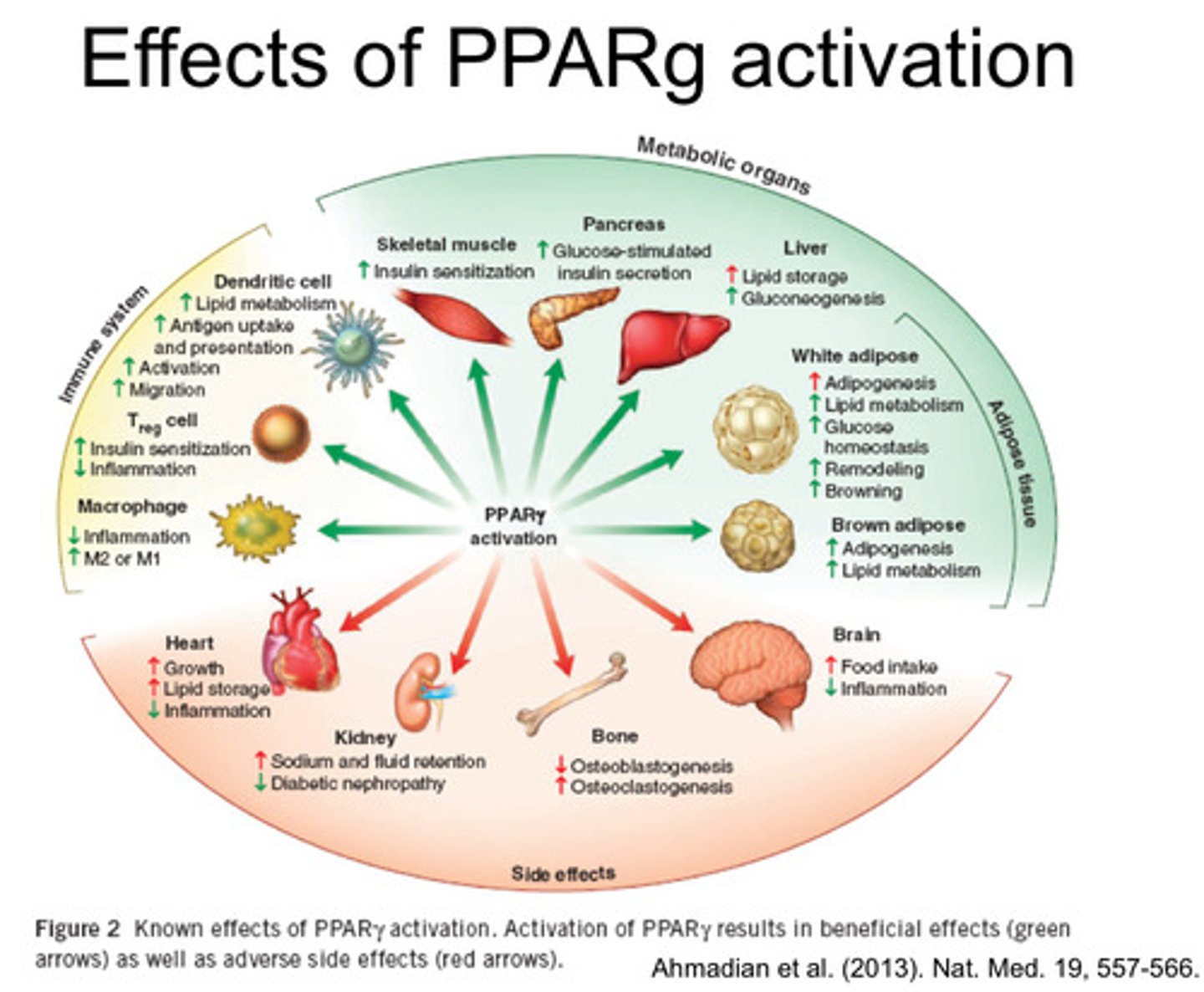
What are the effects of PPAR-gamma activation?
§ Activates transcriptional genes in glucose homeostasis and lipid metabolism.
§ Causes changes in lipidgenesis
§ Increases glucose uptake in muscle and adipose tissues.
§ Reduces insulin resistance in peripheral tissues.
How do a-GLUCOSIDASE INHIBITORS (AGIs) work
•AGIs inhibit the absorption of carbohydrates from the small intestine
•Inhibit Glucosidase enzymes that convert complex non-absorbable carbohydrates into absorbable ones (effective in Type I and Type 2).
•Reduce postprandial hyperglycaemia.
Side effects: flatulence and diarrhoea
Acarbose and Miglitol
How do AMYLIN ANALOGUES work
Amylin (37aa) main component of pancreatic amyloid related to calcitonin/CGRP:
Decreases gastric emptying
Inhibits glucagon release
Promotes satiety, decreases food intake
—
-Related to beta-amyloid, which forms aggregates in neurodegenerative diseases
Example: Pramlintide - analogue of human amylin with Pro replacement as in rat amylin - does not aggregate
-Can be used in both TYPE I and II
INSULIN
-See insulin quizlet
Decision cycle for management of Type 2 diabetes
What are the agents for obesity?
- lipase inhibitor e.g. Orlistat
- GLP-1 agonist e.g. Buproprione, Naltrexone, Saxenda
-5HT-2C agonist e.g., Lorcaserin