How far?
1/6
Earn XP
Description and Tags
How can the extent of a reversible reaction be influenced?
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Characteristics of physical and chemical equilibrium
A closed system
There is no change in macroscopic properties.
Reversible reactions
Chemical reactions that go in both directions.
the extent of a reaction
How far a reaction proceeds in the forward or reverse direction.
Physical equilibrium
The equilibrium that exists during a physical change when the rate of the forward process is equal to the rate of the reverse process.
Chemical equilibrium
A dynamic process consisting of forward and reverse reaction proceeding at equal rates.
Equilibrium is referred to as dynamic
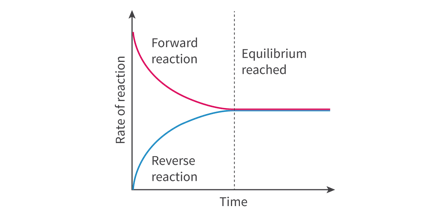
Reactants are sealed together in a sealed reaction vessel (a closed system).
They react together to form the product, therefore the concentration of products increases, and the concentration of reactants decreases.
Once the concentration of the products starts to decrease, the reverse reaction starts taking place
Eventually the rate of the forward reaction becomes equal to the rate of the backward reaction.
System reaches equilibrium.
Equilibrium is referred to as dynamic because both thee forward and the reverse reactions are still taking place.
Concentration of reactants and products
Forward reaction proceeds: concentration of reactants decrease as they react together to form the product. Product concentration increases.
Reverse reaction