Colloids + Suspensions
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
85 Terms
What is a dispersed system
A system in which one component is dispersed as particles OR droplets (dispersed phase) throughout another component (continuous phase)
2 phase system (compared to solutions which only have 1 phase)
E.g. colloids, suspensions, emulsions
Compare a dispersed system to a solution
Dispersed systems have 2 phases, solutions have 1 phase
Disperses systems consist of particles or droplets, solutions consist of molecules
What is a liquid in gas dispersion called?
Liquid aerosol e.g. cloud
What is a solid in gas dispersion called?
Solid aerosol e.g. smoke
What is a gas in liquid dispersion called?
Foam e.g. Bath foam
What is a liquid in liquid dispersion called?
Emulsion e.g. milk
What is a solid in liquid dispersion called?
Suspension e.g. calamine lotion
What is a liquid in solid dispersion called?
Solid emulsion e.g. ice cream
Define colloidal dispersions
Dispersions in which the size of the dispersed particles in the continuous phase is in the range of 10-9 - 10-6m
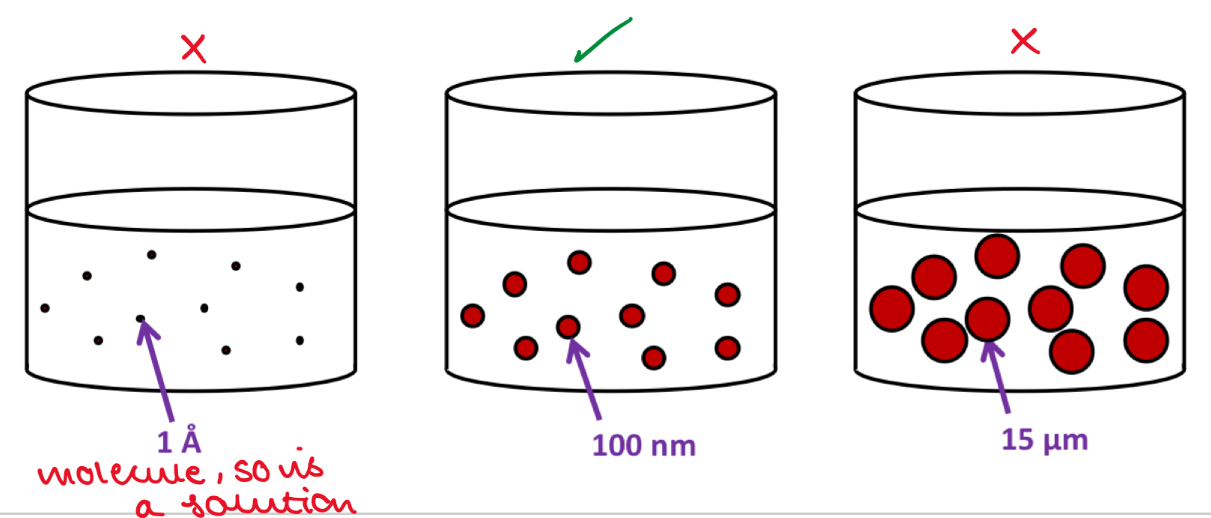
Suppose we add a powder of drug to water. How do I know if I have a colloid or solution?
In a solution, the drug molecules will be dispersed on a molecular scale in the solution - each molecule is separate from another
In a colloid, we have particles of a drug - aggregates of many drug molecules
What is the difference between a pharmaceutical suspension and a colloidal system?
In a pharmaceutical suspension, particle size is > 1um
In a colloid, the particle size is < 1um
Why do we use suspensions?
Poorly soluble drugs cannot always be made into solutions
For taste masking - unpleasant tastes may be less noticeable in a suspension than solution
Drug may be more stable if formulated as a suspension instead of solution
» many drugs are esters and can hydrolyse easily if in a solution
» as a suspension, most of the drug molecules are in the middle of a larger particle so water can’t reach them = less likely to be degraded
Drug may be more stable as a solid, so the suspension is made just before dispensing
Examples of OTC suspensions
Calamine lotion
» Applied topically
» Treats minor rashes / irritation on the skin
Kaolin mixture BP
» Administered orally
» Treats mild diarrhoea
Example of prescription suspensions
Nyastatin suspension
» Administered orally
» Treats fungal infections in mouth, throat, intestines
Betoptic
» Ophthalmic administration
» Reduces pressure in the eyeball
What makes a good suspension?
The suspension must be easy to disperse upon shaking » redispersibility
» A fresh suspension is made consisting of solid + liquid
» Over time the particles will sink to the bottom as particles are more dense than water
» Upon shaking, the particles should become evenly distributed (homogenous)
The suspension should contain particles which are small and of the same size
» Ensures patients do not find it gritty
The suspension must be homogenous
» After shaking and removing the dose, the particles must be evenly distributed throughout the liquid
» Ensures there is an equal concentration of drug throughout the suspension
» So the patient gets the same dose every time
Optical properties (effect of light) of colloids + suspensions
What happens when light passes through a true solution?
What happens when light passes through a colloid / suspension?
If a beam of light passed through a true solution, there is very little scattering of the light, so the path of the beam cannot be seen
If a beam of light is passed through a colloid/suspension, the particles scatter the beam of light so you can see its path

Optical properties (effect of light) of colloids + suspensions
Label the journey of light through a colloid / suspension
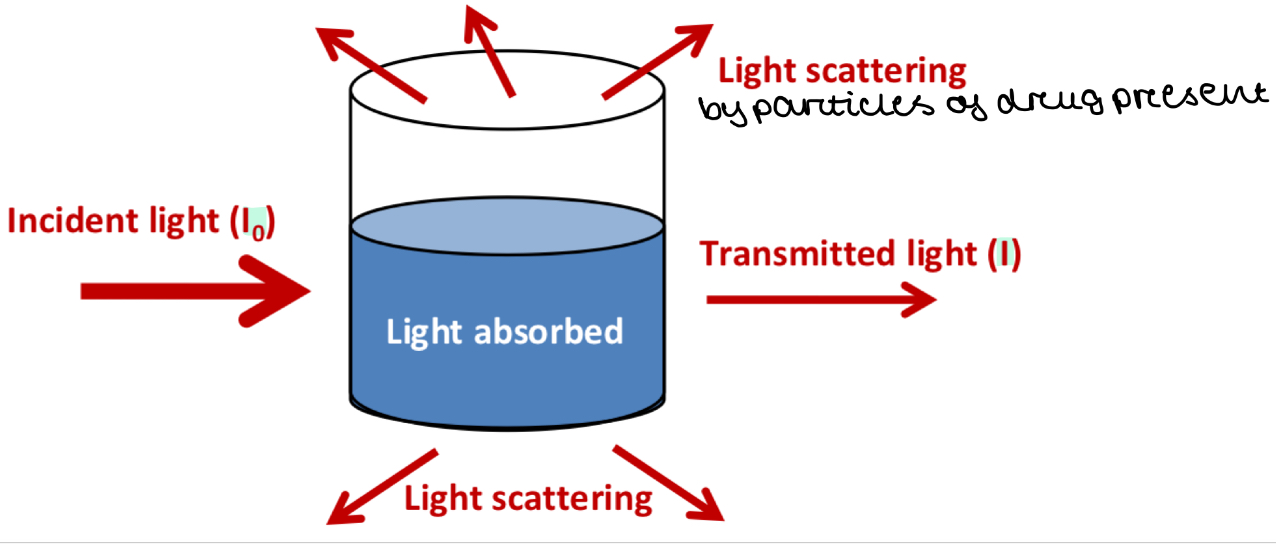
Optical properties (effect of light) of colloids + suspensions
What is the Tyndall effect?
Allows colloidal systems / suspensions to be assessed based on how a beam of light behaves when it comes into contact with the system
Light scattering makes colloidal systems look cloudy or turbid
Turbidity is given by:
I = I0e-tL OR ln(I / I0) = -tL
Transmitted light = Incident light x exponential function-turbidity x path length
The less light that passes through the sample = the more turbid it is = the greater concentration of the dispersed phase (as more particles are scattering the light)
Optical properties (effect of light) of colloids + suspensions
Why is I (transmitted light) always less than I0 (incident light)
Light is scattered by particles in the colloid/suspension
So less light exits the suspension than entered it
Motion in colloids
What kind of motion do the particles undergo?
The particles are small <1um
This means they undergo brownian motion » random movements of the particles in an irregular and zig zag pattern
This is due to random collisions with the solvent molecules, other particles and the container wall
Diffusion in colloids + suspensions
How do the particles diffuse?
How can we calculate factors affecting diffusion?
Particles diffuse from high concentration to low concentration
This means we don’t have to be careful about evenly distributing the solid when making the suspension
Fick’s first law:
dm / dt = -DA x dC/dx
dm/dt = mass diffusing / time
D = diffusion coefficient
A= area across which diffusion occurs
x = distance travelled
dC/dx = concentration gradient
Sedimentation in colloids + suspensions
How do we calculate the velocity of sedimentation of solid particles in a suspension?
Stokes’ Law:
V = 2a2g(σ - ρ) / 9η
a = radius of the solid particles
σ = density of the solid
ρ = density of the liquid
η = viscosity of the liquid
g = acceleration due to gravity (9.8)
Sedimentation in colloids + suspensions
How do you calculate the sediment ratio
R = height of sedimented layer (h) / initial height of suspension (h0)
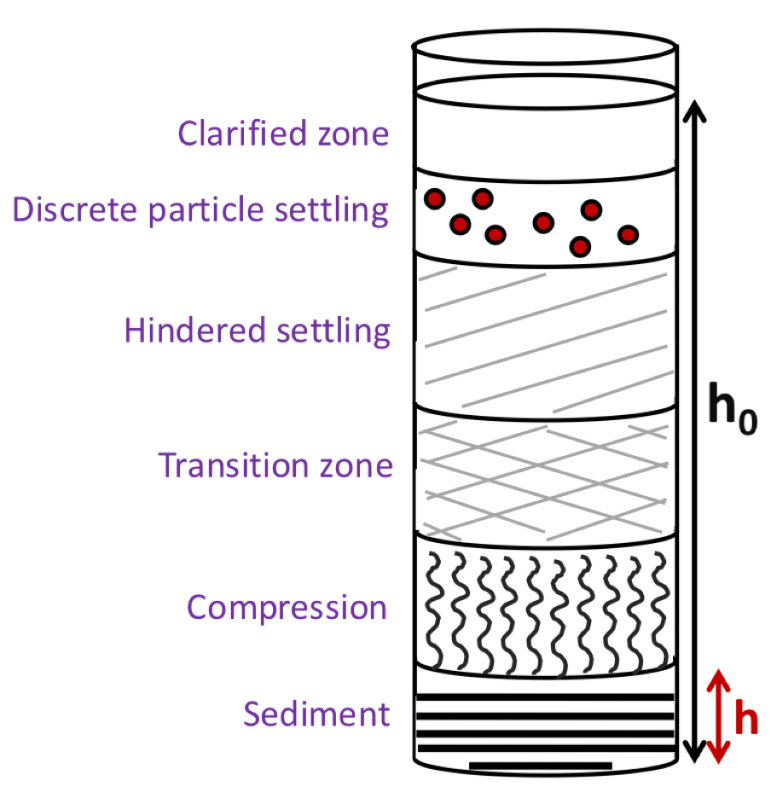
Sedimentation in colloids + suspensions
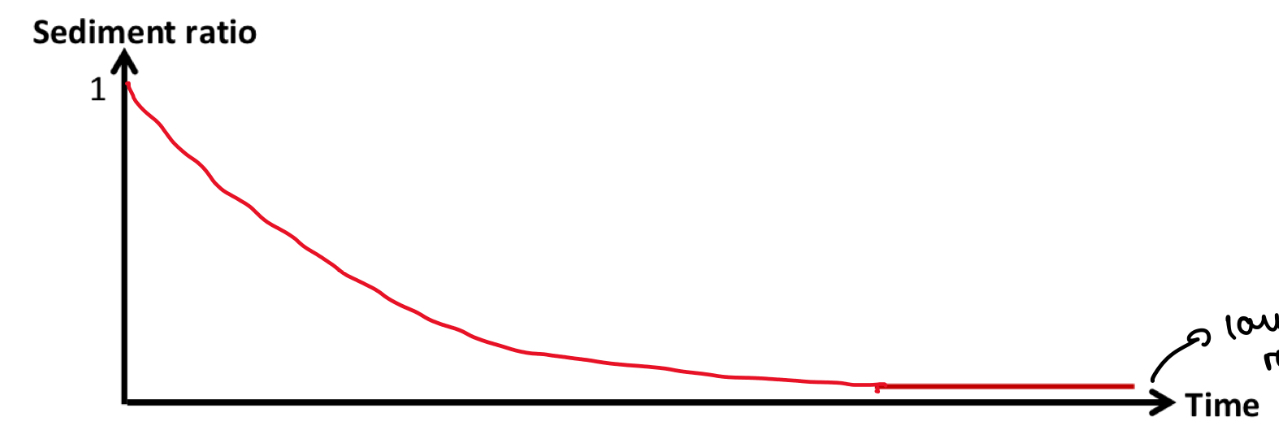
Why does sediment ratio (R) always start at 1?
As at the start, the drug is evenly distributed throughout the suspension
Sedimentation in colloids + suspensions
What happens to sediment ratio over time?
Overtime, there is more sedimentation
So h (height of sedimented layer) gets smaller as the layer of sedimented solid becomes denser
So h / h0 decreases
So sediment ratio decreases over time
A low sediment ratio = caking
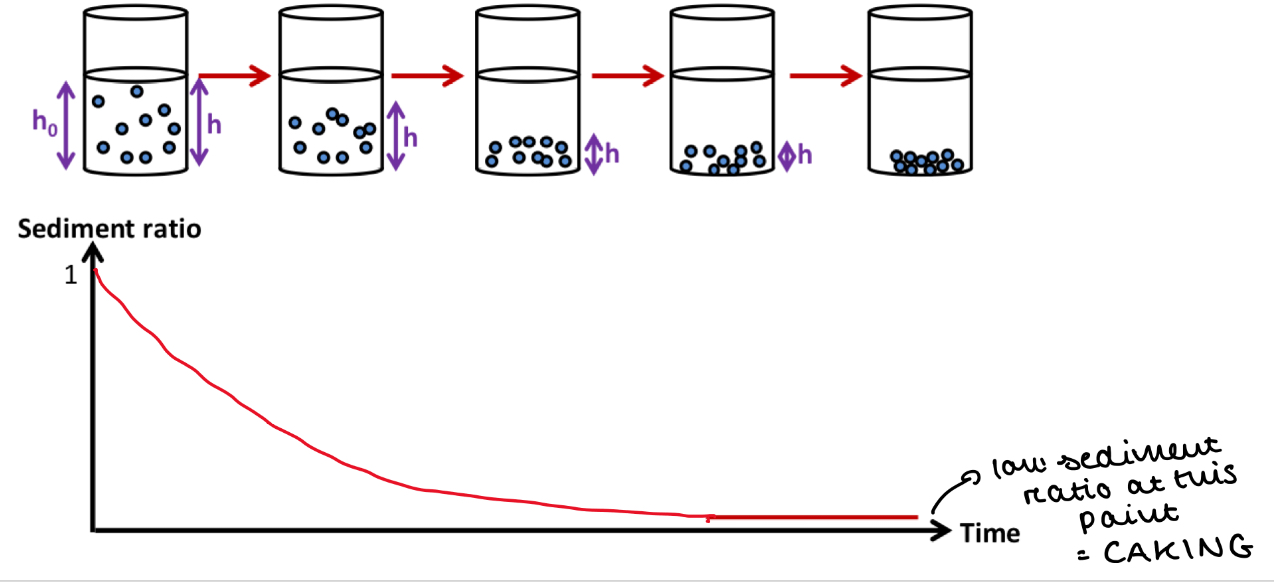
Sedimentation in colloids + suspensions
What can sedimentation of the solid particles lead to?
Caking
Sedimentation can lead to a very dense, non-dispersible aggregate at the bottom of the container
This solid cannot be redispered upon shaking = undesirable for a suspension
How do we make a pharmaceutical suspension?
Drug must have small particles of uniform size
If the drug is water-insoluble, add a wetting agent. This breaks the interfacial tension, ensuring the solid particles disperse easily throughout the liquid
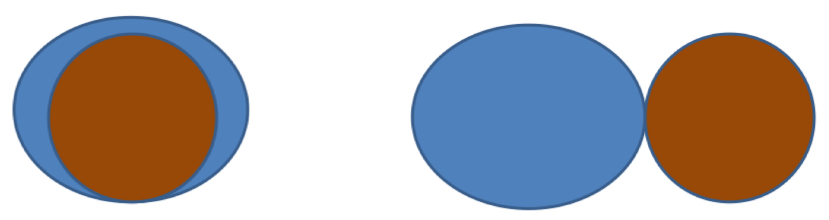
What is interfacial tension?
The energy barrier which prevents the liquid spreading around the solid
If the drug and solvent are hydrophilic = low interfacial tension = liquid spreads around the particles = good suspension
If drug is hydrophobic = high interfacial tension = liquid does not spread around the particle = bad suspension
Wetting agents
What do wetting agents do?
Breaks the interfacial tension, allowing the solid particles to disperse easily throughout the liquid
Wetting agents
Types of wetting agents
Surfactants » have a hydrophilic head and hydrophobic tail
Hydrophobic colloids
Simple solvents e.g. alcohol, glycerol
Wetting agents
How do wetting agents cause particles to disperse throughout the liquid?
Hydrophobic drug particles will stick together. This is called clumping
Wetting of the drug leads to a decrease in surface tension
This prevents the particles from clumping and causes them to disperse throughout the liquid
Hydrophobic drug particles also tend to cling to the container to be as far away as possible from the hydrophilic solvent
Wetting agents decrease adsorption of particles to the container by applying a repellant coating to the particles
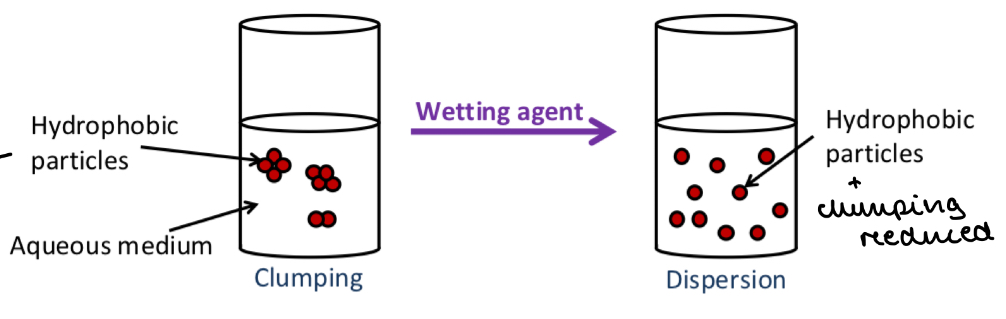
Wetting agents
Describe how a surfactant can be used as a wetting agent
The hydrophobic tails stick to the hydrophobic drug particle
The hydrophilic heads are on the outside
So water can spread around the drug particle to form a suspension
What 2 types of suspensions can we have?
Deflocculaed
Flocculated
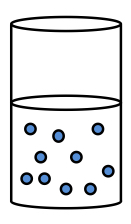
Deflocculated suspension
What is it?
How does the suspension look?
What is the sedimentation like?
Good or bad?
The particles remain as separate units
Suspension remains cloudy for a prolonged period of time
Small volume of final sediment
Rate of sedimentation is slow
» This prevents liquid from being trapped in the sediment
» So the sediment becomes compact, causing caking
» Difficult to redisperse = bad suspension
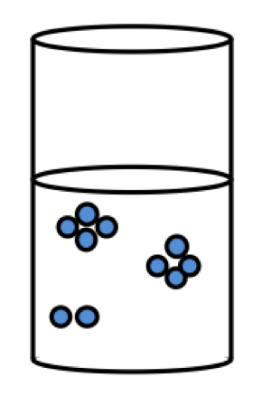
Flocculated suspension
What is it?
How does the suspension look?
What is the sedimentation like?
Good or bad?
The particles exist as loose aggregates
Suspension clears quickly
Large volume of final sediment
Rate of sedimentation is rapid as the aggregates are heavier
» This leads to liquid entrapment within the sediment
» So easy to redisperse = good suspension
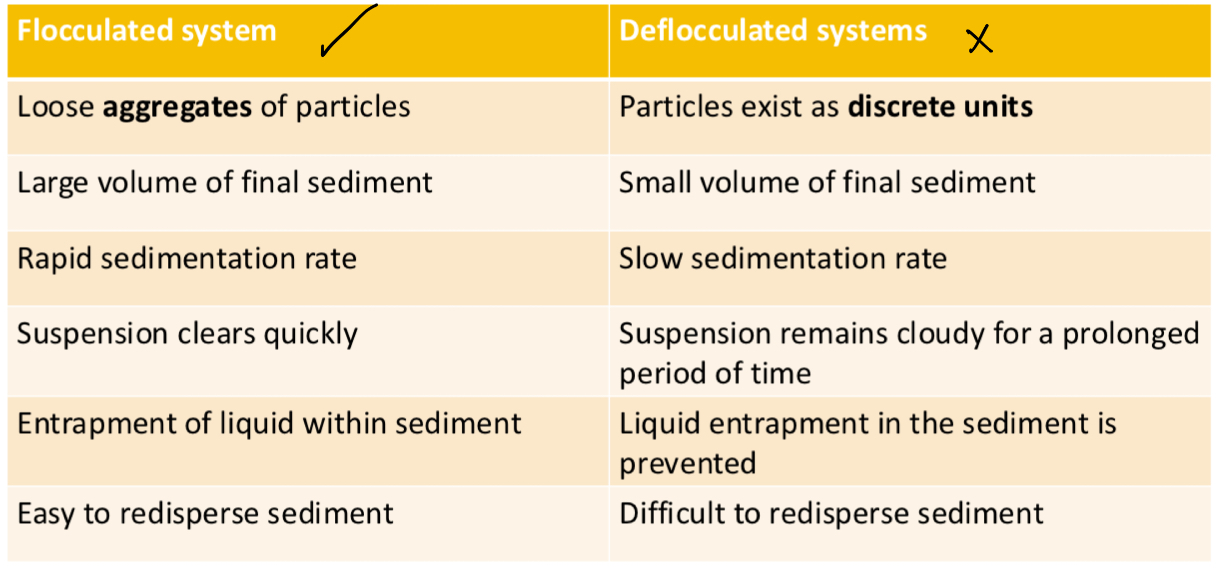
Compare a flocculated and deflocculated suspension
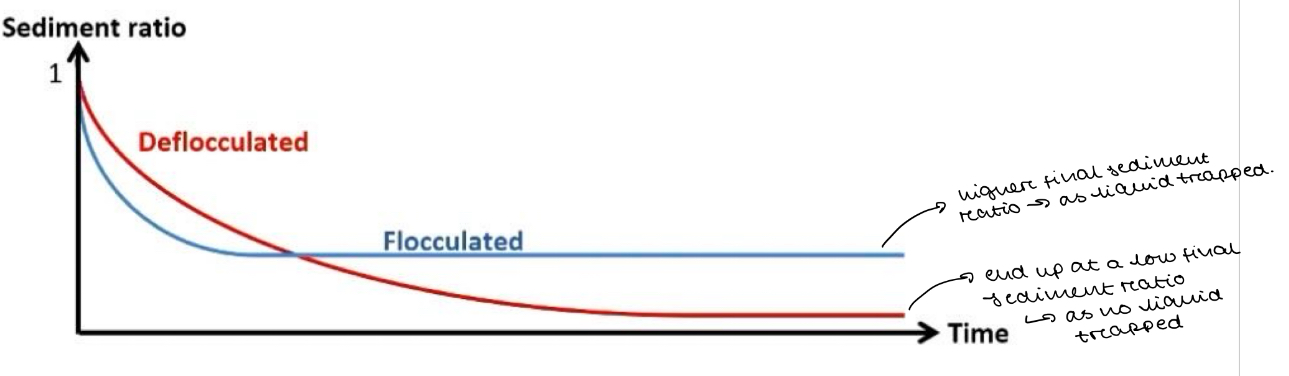
Draw a graph to show how the sediment ratio differs in a flocculated and deflocculated suspension
Draw a diagram showing how the different states of a suspension arise
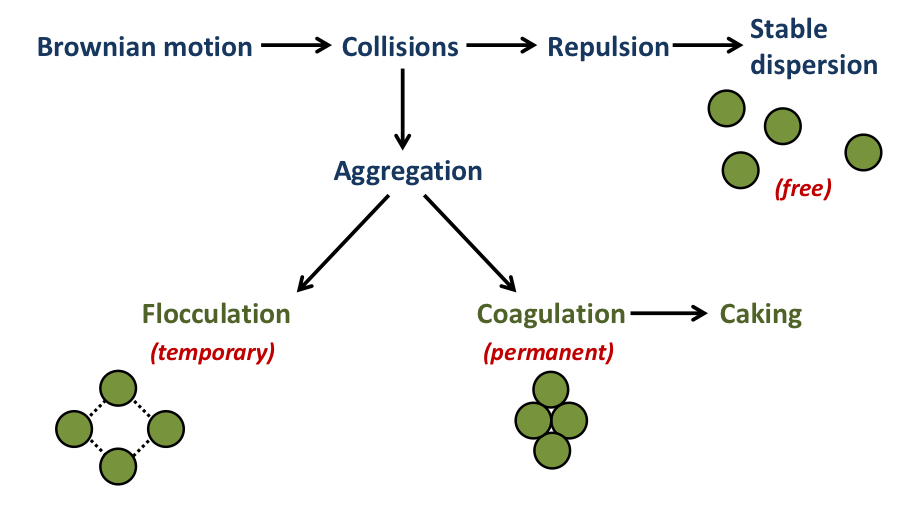
What is the difference between flocculation and coagulation in a suspension?
Coagulation:
Arises when the particles are closely aggregated and difficult to redisperse
Strong forces holding the particles together
Leads to caking
Flocculation:
The aggregates have a loose structure in which the particles are a small distance apart
The particles are only weakly bound together
What is caking?
The formation of a densely packed, non-dispersible, aggregate at the bottom of the container in a suspension
This means that the drug is not evenly distributed throughout the suspension
Which can lead to the patient underdosing and then overdosing (as all the drug is at the bottom)
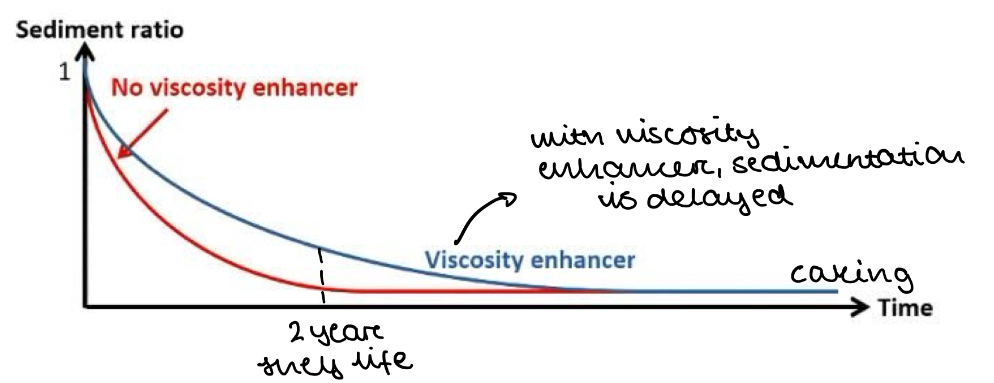
How can we delay sedimentation and caking?
Viscosity enhancing agents
V = 2a2g(σ - ρ) / 9η
So if we increase viscosity of the liquid phase (η), the rate of sedimentation is reduced
E.g. polysaccharides, celluloses (presence of polymers makes a suspension thicker)
E.g. hydrated silicates, carbomers and silicon dioxide
NOTE: This only DELAYS sedimentation. It does not STOP it, so caking will still happen eventually
Draw a graph to show how the sediment ratio changes when a viscosity enhancing agent is added to a syspension
How can we prevent caking?
By using flocculating agents
Flocculating agents
How do flocculating agents prevent caking?
Encourages the formation of flocs
These are loosely bound aggregates which sink quickly
This means they have no time to pack together tightly
So there is liquid trapped between the particles
So the particles can be dispersed easily
Flocculating agents
Ideally, how flocculated do we want the system to be?
Partially deflocculated
Too deflocculated = caking
Too flocculated = sinks too quickly before patient can take medicine
Flocculating agents
Examples
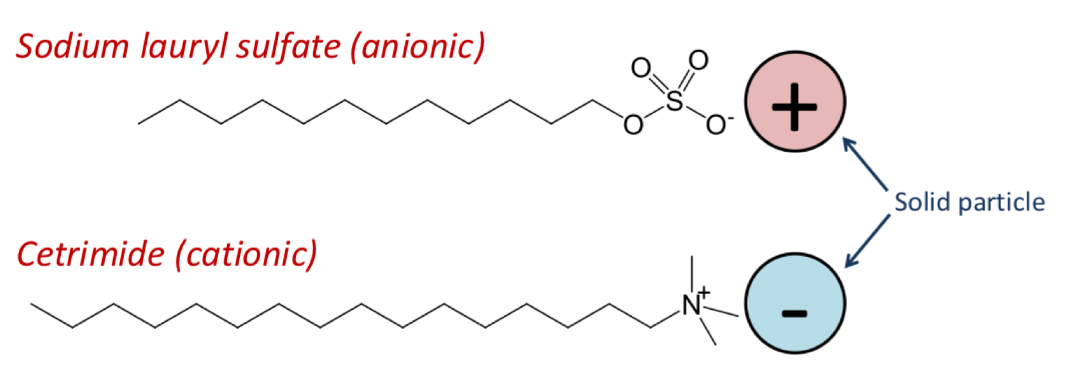
Electrolytes AKA salts (sodium acetate, phosphate, citrate)
Surfactants (ionic or non-ionic)
Polymers (starch, cellulose, alginates)
Carbomers or silicates
Electrical properties of particles in colloids and suspensions
Particles have a surface charge
In water, this is typically negative e.g. COOH will deprotonate to form COO-
The negative charge at the particle surface will attract positive ions in solution
These will then attract negative ions
This forms an electrical double layer
How do all the forces in a colloid/suspension work?
All the particles in a suspension are made of the same drug so have the same charge » these particles repel each other (VR)
There are also some attractive forces (VA) from Van der Waals forces between the molecules
Permanent dipole - permanent dipole interactions
Permanent dipole - induced dipole interactions
Temporary dipole - induced dipole forces
Flocculating agents
What theory does flocculation depend on?
DVLO theory:
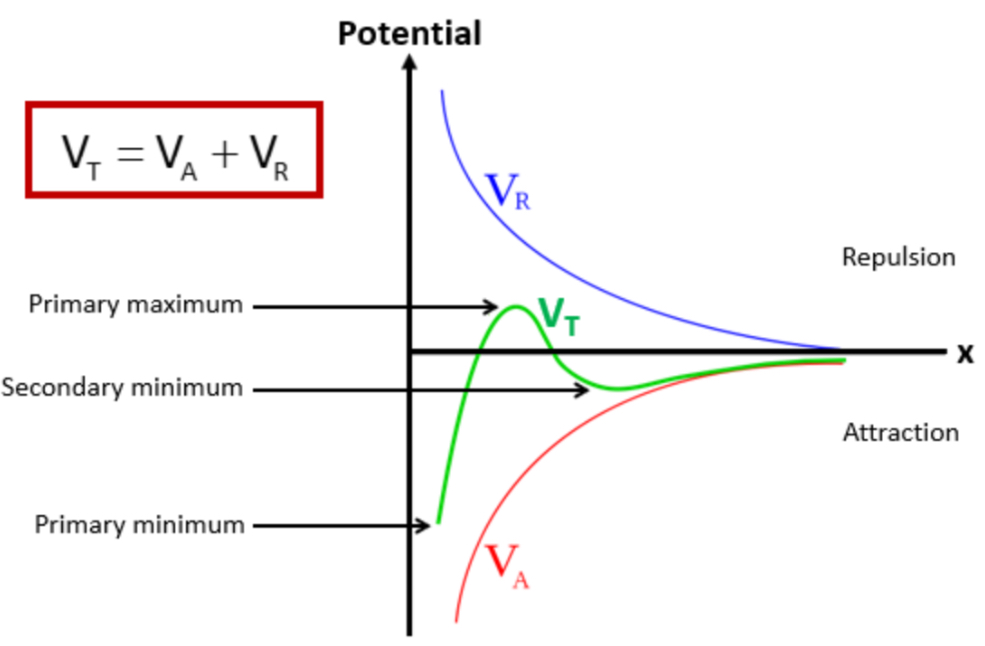
VT = VA + VR
VT = total potential energy of interaction
VA = potential energy of attraction (VDW forces between molecules)
VR = potential energy of repulsion (repulsion between particles)
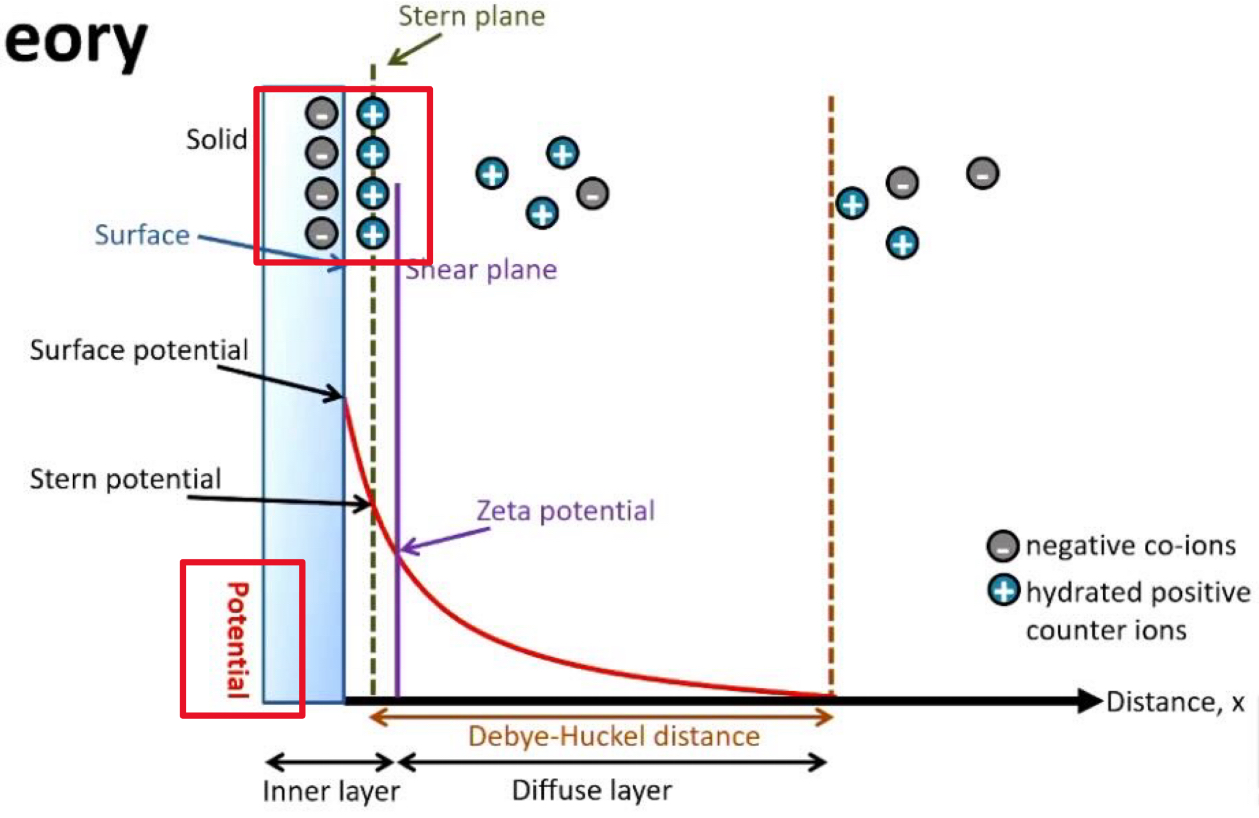
Explaining DVLO Theory
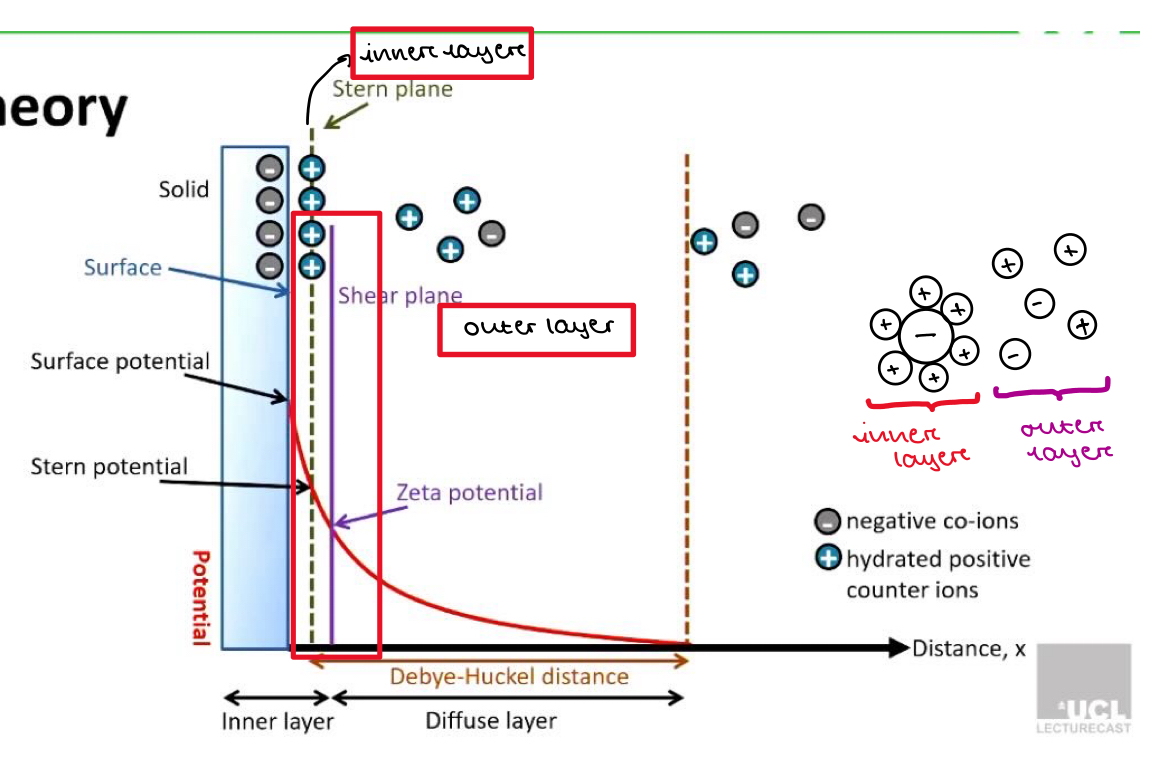
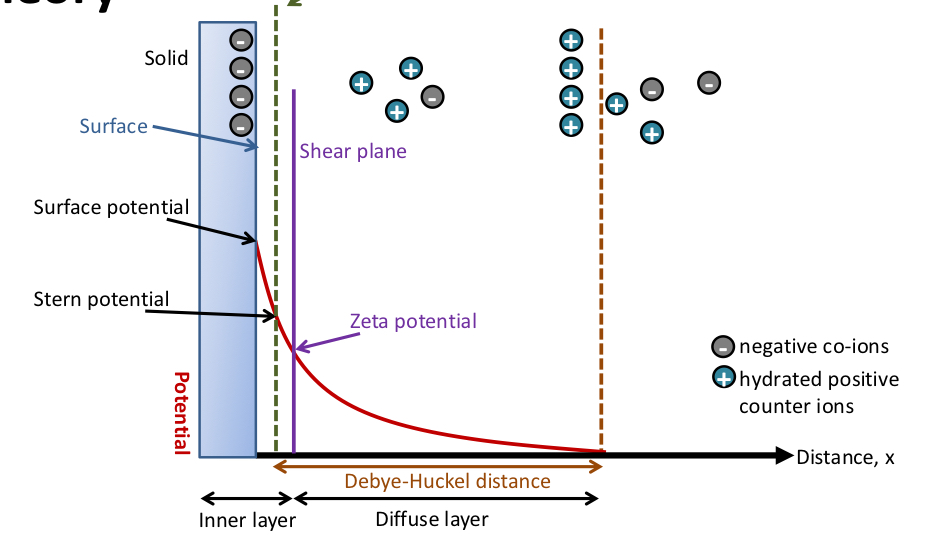
Stern layer
Potential
There is a negative charge at the surface of the particle
So positive counter ions in solution will be attracted to the surface of these particles very strongly.
These are tightly attached and don’t move
This is called the stern layer
Potential shows the charge between 2 adjacent particles
When particles are close together = more repulsion = more potential
Explaining DVLO Theory
Shear plane
Double layer of the particle surface consisting of the inner layer (stern layer) and outer layer (diffuse layer)
Inner layer (stern layer):
Ions are tightly bound to the the surface of the particle
Strong interactions
Ions move with the particles
Outer layer (diffuse layer):
Ions are less tightly bound and are constantly in motion
Weaker interactions to the particle surface
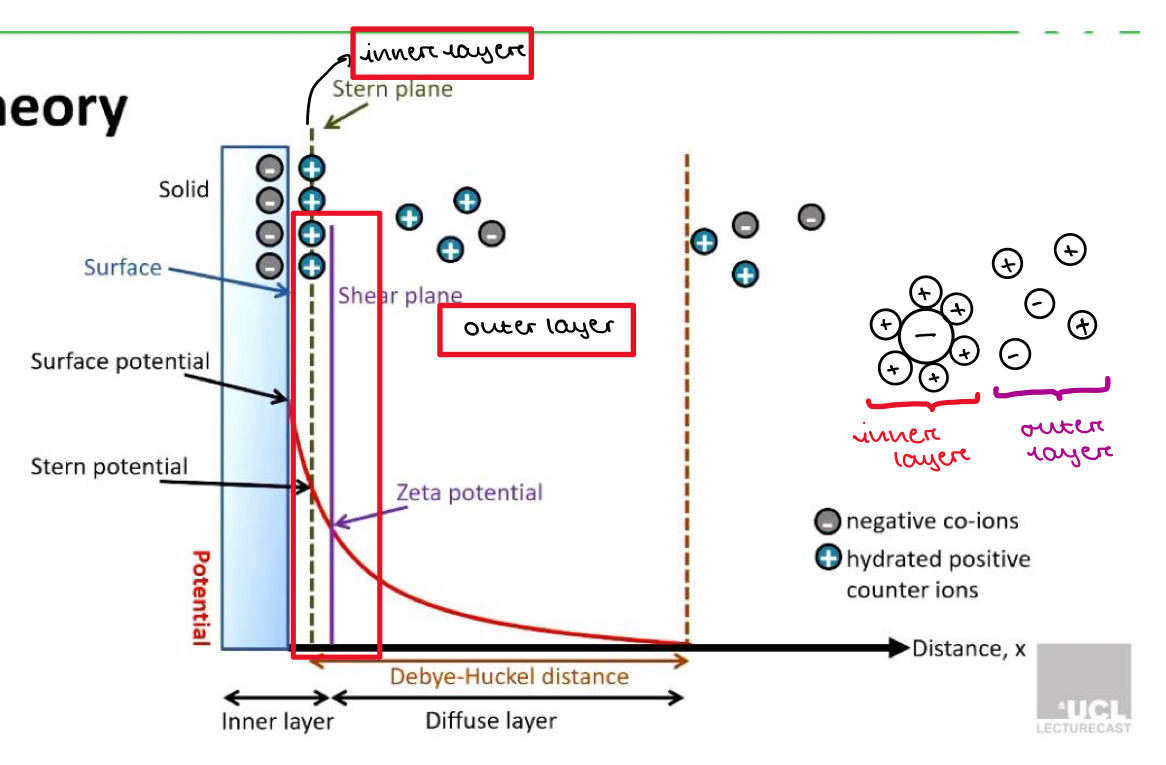
Explaining DVLO Theory
Zeta potential
Shows the difference in electrical potential between the inner layer (close to the particle) and outer layer (further away from the particle)
Tells is how strong the repulsive forces (VR) are that push other particles away
Very positive or very negative zeta potential:
Large difference in charge between inner and outer layer
Strong repulsive forces so particles more likely to stay apart and not stick together
Good stability
Zeta potential close to 0
Difference in charge between inner and outer layer is small
Minimal repulsive forces so particles are more likely to be attracted to each other and clump
Poor stability
Explaining DVLO Theory
Why does potential fall to 0 after the Debye-Huckel distance?
As we move away from the particle surface, the number of counter-ions decreases and the number of same charge ions increases
When we get a certain distance from the particle (the edge of the diffuse layer) there is an equal number of positive and negative ions
So there is a potential of 0 after the Debye-huckel distance
Explaining DVLO Theory
How does this link to flocculation
VT = VA + VR
For flocculation to happen, the attractive forces VA must be strong enough to overcome the repulsive forces VR
So we must decrease VR
We cannot control VA
However we can control VR. VR depends on:
The surface charge
The thickness of the double layer (distance over which potential falls to 0)
In a potential x distance graph plotting VR, VT and VA, label the primary maximum, secondary minimum and primary minimum
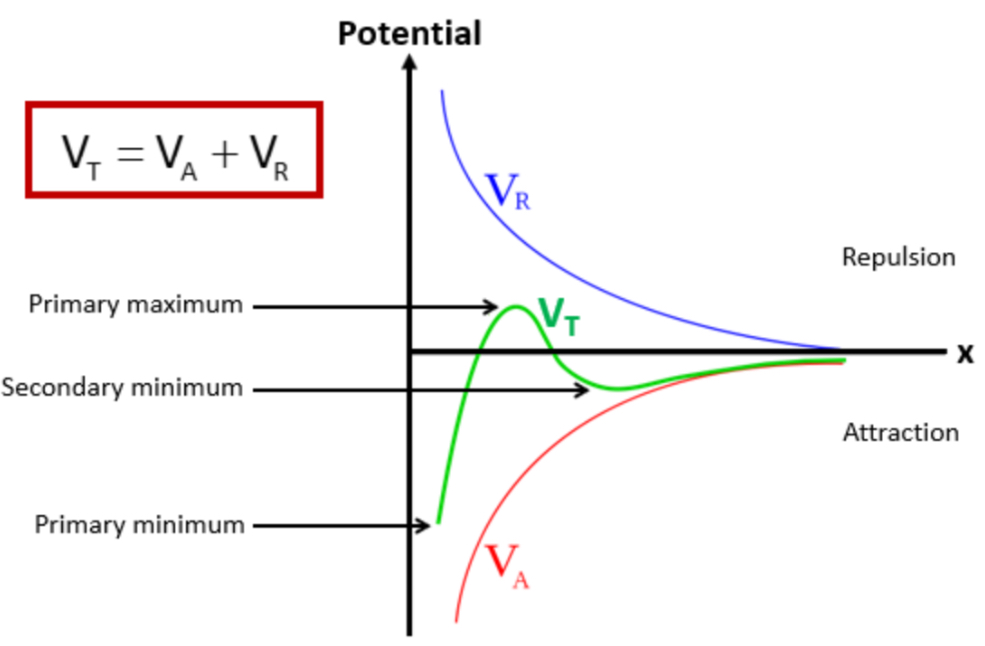
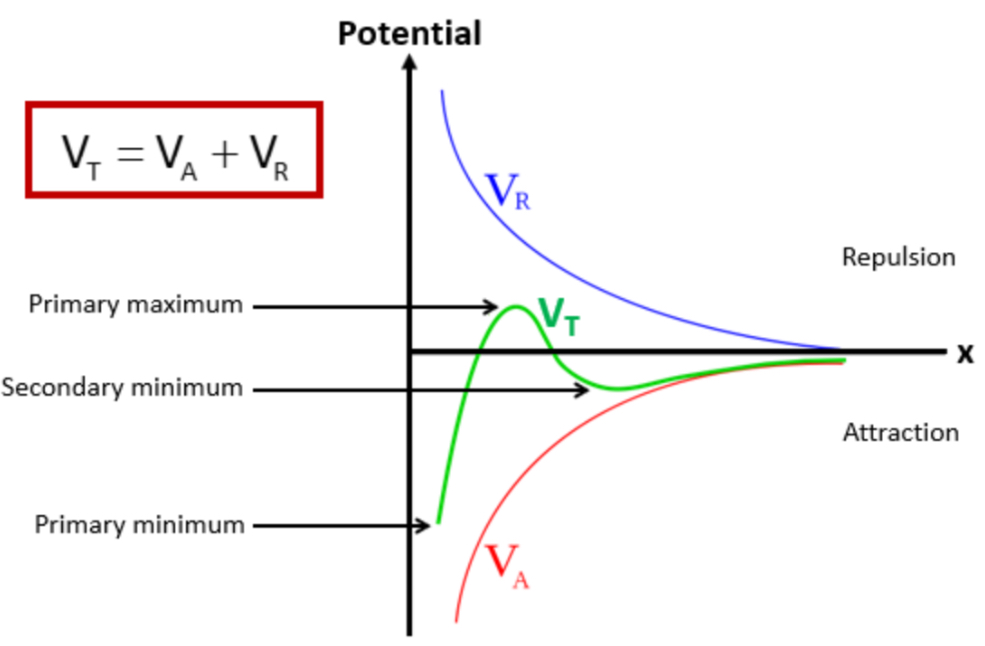
What happens at the primary minimum?
Caking » the particles a small distance away from each other
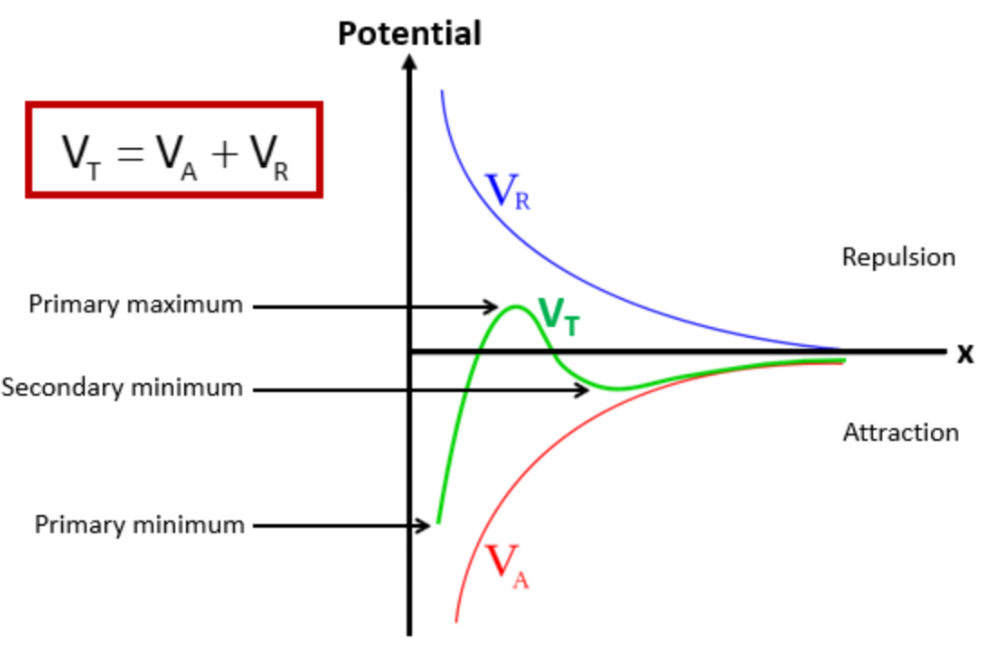
What happens at the secondary minimum?
Flocculation » this is the ideal distance we want the particles to be away from each other
What determines the stability of the colloidal system?
When particles are close together, attractive forces dominate
This gives an energy minimum
Which causes clumping
When particles are slightly further away from each other , they experience repulsive forces due to their electric double layers
This creates an energy maximum
Which acts as a barrier to prevent particles from coming too close, so particles remain dispersed
If the energy maximum is:
Large » particles stay dispersed
Small » particles will aggregate
Methods to prevent caking
Add an electrolyte
Add a surfactant
Add a polymer
What happens to a suspension when you add an electrolyte ie. Counter anions?
Increases the Debye-huckel parameter (K) » as increases the number of charged particles in the solution
Decreases the thickness of the electric double layer (1/K) » as it is inversely proportional to K
Decreases zeta potential » counterions neutralise the surface of the particle = less repulsion = smaller difference in potential
Increases the depth of the secondary minimum » repulsive forces reduced = particles more able to come close to each other = deeper secondary minimum
Deeper secondary minimum = stronger flocculation
Compare the sedimentation in a suspension when:
No electrolyte is addded
Electrolyte is added
Too much electrolyte is added
No electrolyte
High zeta potential
Strong repulsion between particles
Slow sedimentation = low sediment ratio
Prevents liquid being trapped in the sediment
Densely packed
Caking
Electrolyte
Zeta potential decreases
Repulsive forces between particles decreases
Rapid sedimeintation = high sediment ratio
Liquid trapped in sediment
Flocculation
Too much electrolyte
Completely neutralises the surface charge on the particles
Zeta potential decreases too much and becomes negative
Repulsion between particles is too weak
Leads to irreversible aggregation of particles
Caking
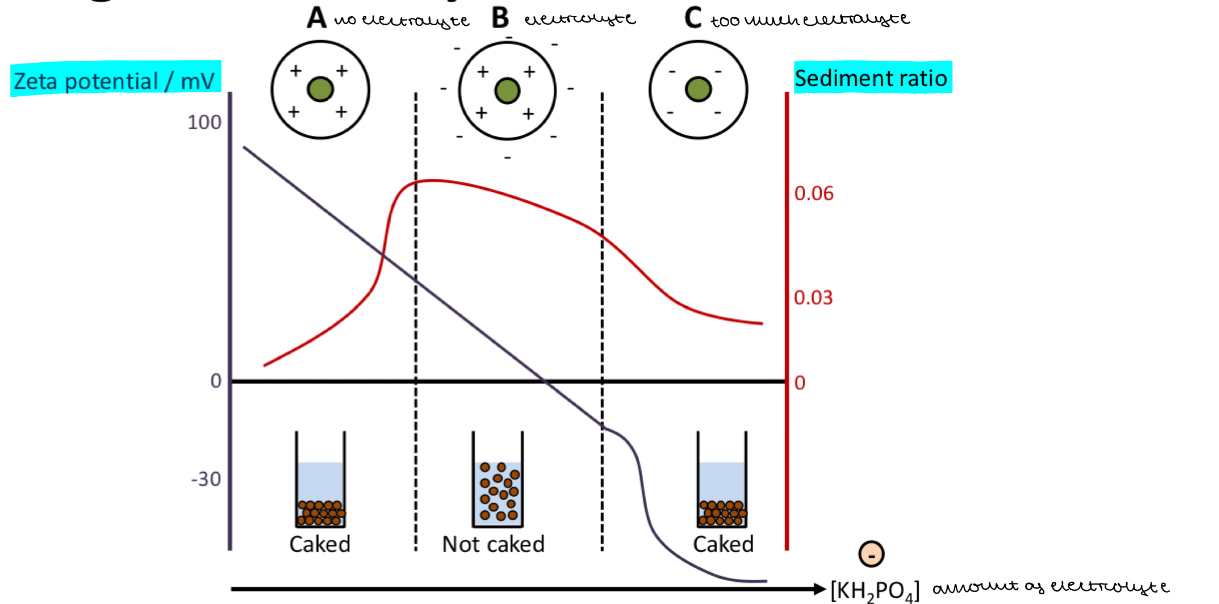
As you add more electrolyte what happens to zeta potential
MORE ELECTROLYTE = ZETA POTENTIAL DECREASES
What zeta potential is ideal and does flocculation occur at?
WHEN ZETA POTENTIAL CLOSE TO 0
What is the difference between adding an electrolyte to a suspension and adding a viscosity enhancing agent?
Electrolytes prevent caking
Viscosity enhancing agents only delay caking
EQ:
What happens when you add KH2PO4 to a bismuth subnitrate suspension (positively charged surface)?
Initially the suspension is deflocculated
Adding KH2PO4 (K+, H+, PO43-) causes a reduction in zeta potential because the phosphate ions adsorb to the particles
As more KH2PO4 is added, the zeta potential reduces to zero, then turns negative
To obtain a flocculated suspension, need to control the zeta potential by adding the right amount of electrolyte
How does adding a surfactant prevent caking?
Neutralises the surface charge of a particle
So reduces repulsion between the particles
How does adding a polymer prevent caking?
Examples of polymers we can use
Groups in the polymer interact with the surface of the particles
The free end of the polymer attaches to another particle
This causes interparticle bridging leading to flocculation
If there are no other particles to interact with, the free end of the polymer coats the particle. This leads to a deflocculated system
So we have to carefully control the polymer concentration
E.g. starch, alginate
Maalox Suspension
Drug class
Active ingredients
How and where do they act following oral administration?
List the excipients and identify their roles in the formulation
Antacid (neutralises stomach acid)
Aluminium hydroxide and Magnesium hydroxide
Acts in the stomach to neutralise HCl, which raises pH levels
Excipients:
Citric acid » adjusts pH, enhances taste
Peppermint oil » improves taste
Mannitol » sweetener
Domiphen bromide » preservative as lots of sweeteners
Purified water » solvent
What excipient do you need in a suspension that contains water + sweeteners?
Preservatives e.g. domiphen bromide » prevents bacteria growth
What excipients should you always expect in a suspension?
Water
Sweetening agent
Preservative
Thickening agent
Nitrazepam Oral Suspension
Active ingredient
What is it used for?
Where does it act following oral administration?
Which excipient is used to increase viscosity to prevent caking?
Nitrazepam
Used as a sedative to treat anxiety and sleeping disorders
Acts in the brain
Carbocymethyl cellulose (CMC) sodium (thickening agent)
Compare Nitrazepam Oral Suspension and Maalox Suspnesion, in terms of site of action following oral administration
Nitrazepam: Acts systematically on the brain so is absorbed
Maalox: Acts locally in the stomach so does not need to be absorbed
Give 2 formulation measures you could take to reduce the sedimentation rate of solid drug particles in a suspension
Increase viscosity of continuous phase » by adding thickening agents such as carboxymethyl cellulose
Decrease particle size
Stabilising Bismuth Subnitrate by adding Phosphate Ions
What is the flocculating agent
Potassium Phosphate
Stabilising Bismuth Subnitrate by adding Phosphate Ions
How does the addition of phosphate ions prevent caking?
Bismuth subnitrate particles are positively charged
The negative phosphate ions adsorb onto the bismuth subnitrate particles
This reduces the positive charge on the particles
Increasing the phosphate ion concentrations further reduces the positive charge as more anions adsorb onto the particles
Eventually the net charge of the bismuth subnitrate particles becomes zero
Further addition of phosphate ions causes the charge to become negative
This reduces repulsion between particles
Particles aggregate more easily
Increases particle size
Increases settling rate
Decreases caking
Stabilising Bismuth Subnitrate by adding Phosphate Ions
Illustrate with a sketched graph how you expect the sediment volume to change as increasing amounts of phosphate ions are added to bismuth subnitrate suspension
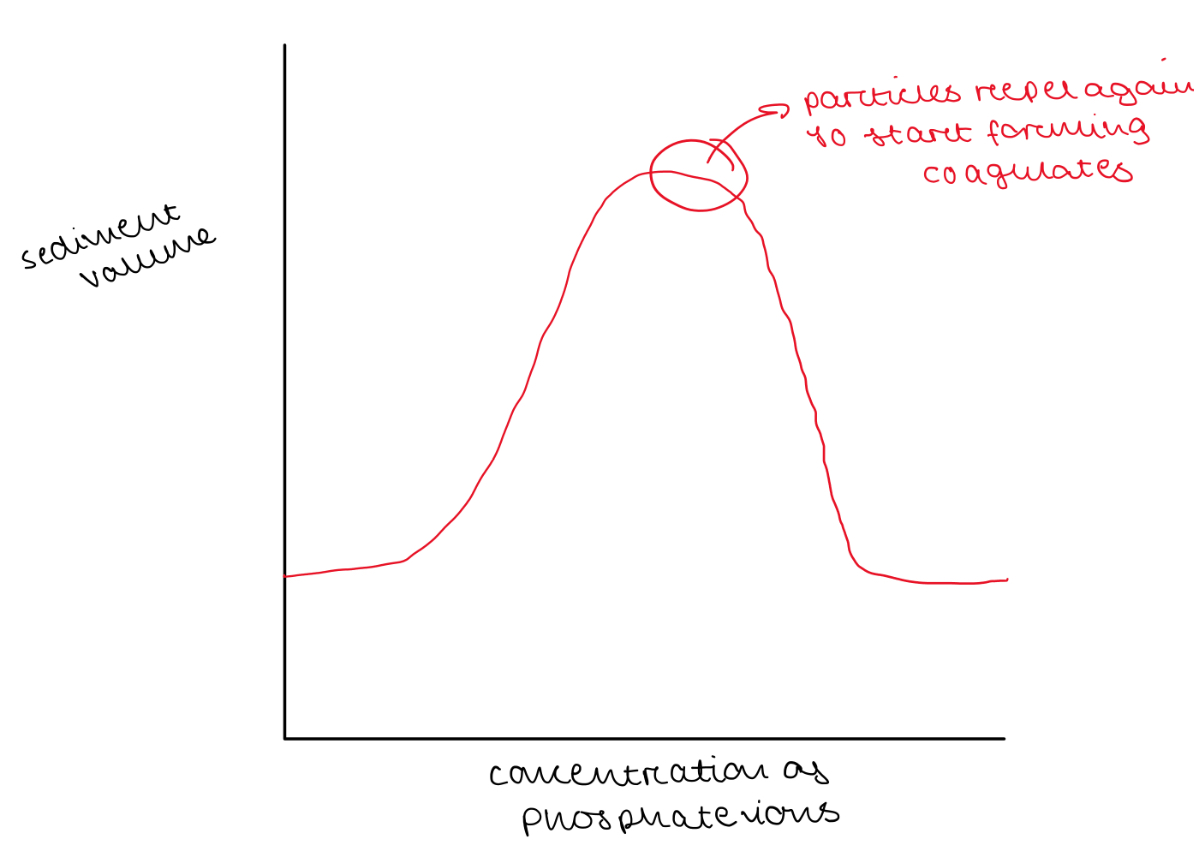
Stabilising Bismuth Subnitrate by adding Phosphate Ions
Method
Prepare suspending mediums from stock electrolyte solution of 320mM KH2PO4 solution
Weigh 10g of bismuth subnitrate in a weighing boats and transfer to 100mL measuring cylinders
Add suspending medium to each, stopper cylinder and shake to disperse bismuth subnitrate particles in liquid medium
Invert cylinder to ensure no solid particles are stuck to the base
Leave suspensions to stand and measure sediment volume the next day
Plot a graph of electrolyte concentration against sediment ratio R
R = volume of sediment / total suspension volume
Preparation of Calamine Lotion
What is the pharmaceutical function of each of these ingredients in the formulation the calamine lotion suspension:
Zinc oxide
Glycerin
Bentonite
Sodium Citrate
Stabilises pH, enhances stability
Increases viscosity
Increases viscosity
Maintains pH, prevents aggregation of particles
A pharmaceutical company sells a medicine comprising ibuprofen particles in an aqueous medium, which they describe as a colloidal dispersion. Given that the particle size is measured to be 2.5um, comment on this description
Not colloidal
Particles too big
Colloid particles must be <1mL
Distinguish clearly between the terms coagulation and flocculation
Coagulation: particles are bound together by strong forces, very close together and often cannot be redispersed » leads to caking
Flocculation: particles are bound together weakly, not as close together, can be redispersed
A pharmaceutical company is attempting to prepare a suspension of a drug D (structure shown).
On their first attempts, the company find that the suspension is poor quality, with significant amounts of clumping and clinging observed. Explain these observations and suggest a possible solution to these problems.
Eventually the company is able to implement a solution to the clumping and clinging problems. However now they find that the suspension tends to cake upon standing. Explain what is meant by the term caking, why it arises and why it is problematic.
The company decide to add K3PO4 to their suspension. Explain why they made this decision, and what affect the K3PO4 will have.
Clumping
Particles stick together closely so minimum surface area exposed to continuous phase
Usually floats to the top
Clinging
Particles cling to the sides of the container
Solution
Add a wetting agent
Caking is the formation of a dense, compact sediment in a suspension that is difficult to redisperse
It occurs when particles in the suspension aggregate and settle at the bottom, forming a hard mass due to strong forces between the particles
» due to inadequate particle stabilization, insufficient repulsive forces, changes in the suspension’s viscosity
Problematic as leads to inaccurate dosing
K3PO4 is an electrolyte
The negative phosphate ions bind to the surface of the positive particles
This decreases the zeta potential
This produces flocs
Easy to disperse
So no caking
Give the mathematical expression for the sediment ratio
R = height of sedimented layer (h) / initial height of suspension (h0)
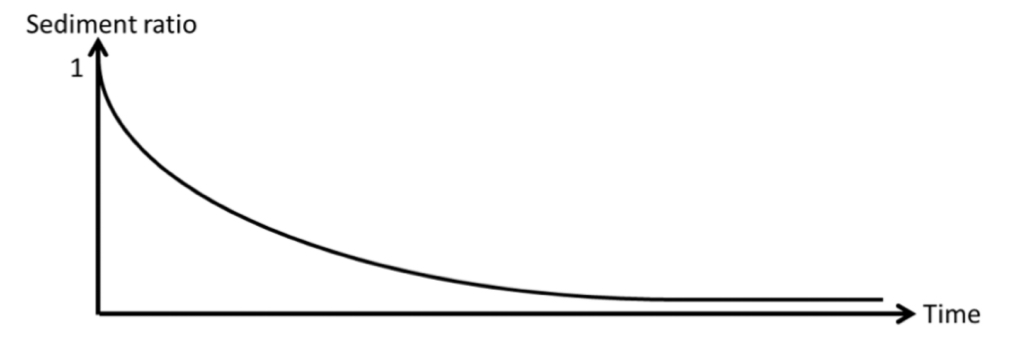
A plot of sediment ratio vs time for a suspension of drug in water is plotted below. Sketch on this plot the curve you would expect if:
(i) A viscosity enhancing agent is added to the suspension
(ii) A flocculating agent is added to the suspension
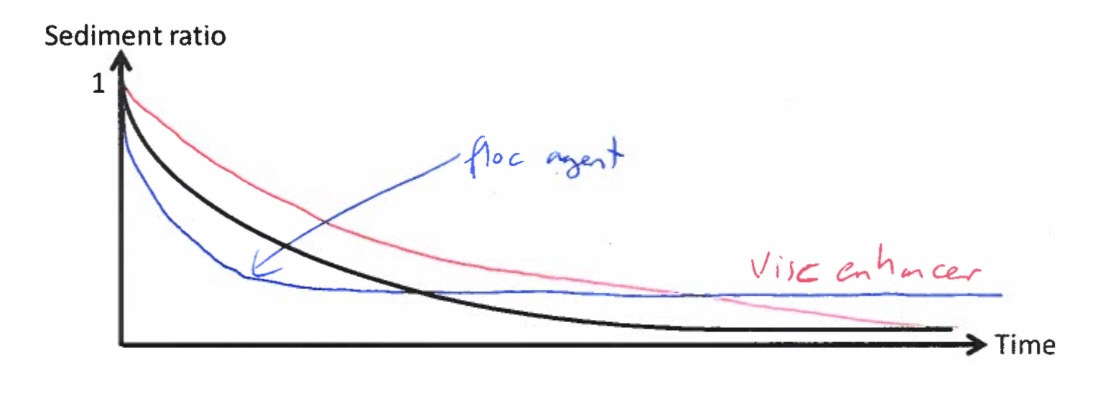
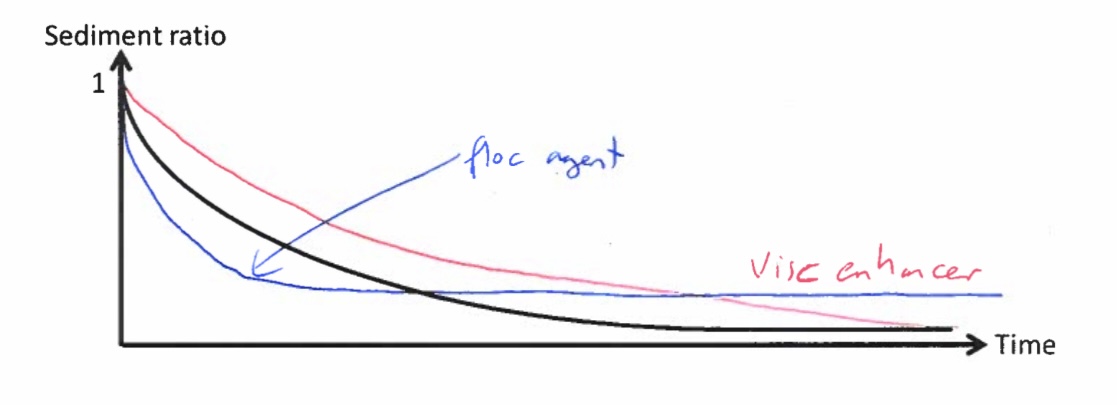
Explain the differences between the sediment ratio vs time plot for the suspension comprising the drug and water alone and that where a flocculating agent is added
Suspension with Drug and Water Alone (No Flocculating Agent):
Sediment ratio decreases gradually over time as particles settle slowly.
Particles form a dense, compact sediment (caking), making redispersion difficult.
The final sediment volume is small due to tight packing of particles.
Suspension with a Flocculating Agent:
Sediment ratio decreases more rapidly at first because flocculated particles settle faster.
Particles form a loose, porous sediment that is easy to redisperse.
The final sediment volume is higher since flocs trap more liquid, preventing compact packing.
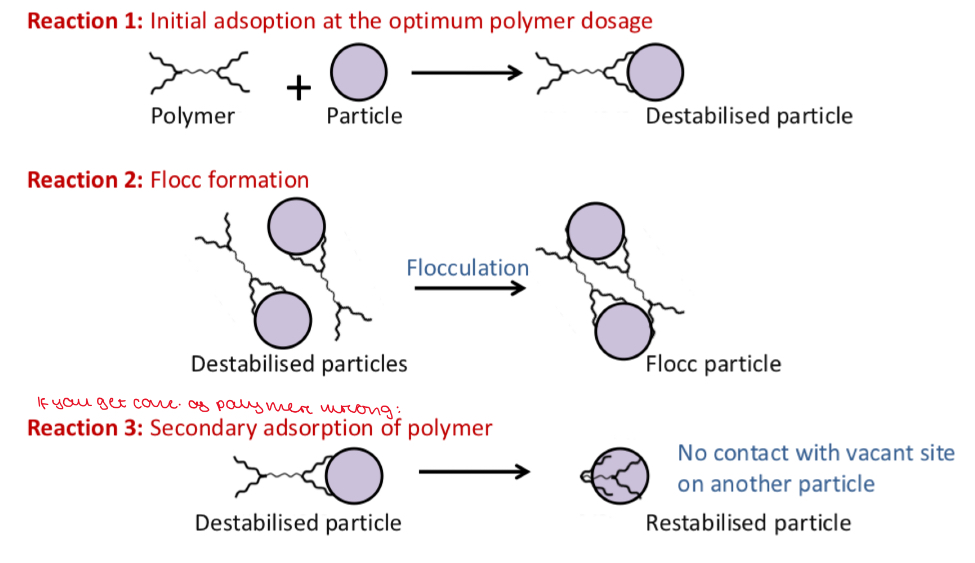
Describe with diagrams how the addition of a polymer to a suspension can help prevent caking. Why is it important to ensure that the correct amount of polymer is added?
Groups in the polymer interact with the surface of the particles
The free end of the polymer attaches to another particle
This causes interparticle bridging leading to flocculation
If there are no other particles to interact with, the free end of the polymer coats the particle. This leads to a deflocculated system
So we have to carefully control the polymer concentration