Chemistry Regents Review: Nuclear Chemistry & Unit 2 (week #5)
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Why aren’t radioactive isotopes included in the calculation of the weighted average?
because they aren’t stable in a natural environment & random breakages of particles being emitted
Transmutation
when an element turns into another
Nucleons
particles in the nucleus
if the ratio of nucleons changes → chemical changes
Half-Life
time required for half of the amount of an isotope to decay
Half-Life Chart
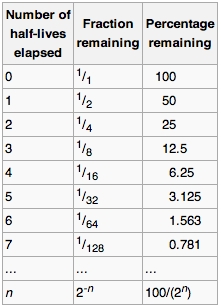
When you heat a radioactive material its half-life…
remains the same
Nuclear Fusion (fuse)
2 smaller and nuclei fuse tgth and form one bigger and heavier nucleus
H & He ONLY
very high temps ONLY
Nuclear Fission
a larger nucleus splits into 2 or ore smaller nuclei
2 ways nuclear fission can occur
spontaneous (by itself)
artificially (absorbing a neutron)
Nuclear Fission and Fusion both take mass and….
convert it to make very large amounts of energy
Radioactivity
radioactive isotopes spontaneously decay over time
As penentration power increases…
ionization decreases
Short Half Lives
good for diagnosing disorders
Similarity of Ionization Energy and Bright Line Spectra
both emit energy
Differences of Ionization Energy and Bright Line Spectra
ionization loses an e-
ionization requires more energy (physically removing an e- from an element while BLS is just moving e- up and down the energy shells)
Relative Abundance
Carbon-14 is used for
dating of once living remains
Cobalt-60 is used for
treatment of cancer
Iodine-131 is used for
treatment and diagnosis of thyroid disorders
Uranium-239 is used for
dating of geological reamins