S1 Intro to IB HL Chemistry
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
Element
a substance that cannot be broken down by chemical means into smaller substances
Compound
2 or more element chemically bonded together and can be seperated through chemical means
Mixture
2 or more substance not chemically bonded together and can be seperated through physical means
Two types of mixture
Homogenous: uniform
Heterogenous: non-uniform
Filtration
used to seperate insolube solid (residue) from filtrate
Distillation
seperate liquids with different melting point
- lower melting point vapourises first then condenses into liquid
Recrystalisation
Purify solid by dissolving in hot solvent (Solid → Liquid)
Crystalisation
Making pure crystals from impure liquids (Liquid → Solid)
Chromatography
seperate mixture of soluble substances e.g Ink
Kinetic Molecular Theory
Solid: Vibrate in fixed position, Fixed Volume
Liquid: Slide past each other, Fixed Volume
Gas: Move freely, Dynamic volume
Kelvin
Measure the kinetic energy of particle
K = C + 273
Deposition
Gas → Solid
Sublimation
Solid → Gas
Atomic number (Z)
number of proton in the nucleus
Atomic mass (A)
Proton + Neutron = Mass
Mass of electron
1/1836
Isotopes
atom of the same element with the same atomic number but different mass (Neutron)
Relative atomic mass formula
A = (% of first mass) + (% of 2nd mass)… / 100
Mass spectrometer
used to determine the relative atomic mass & identifying isotopes
Steps to identify isotopes
Ionisation: Turn into ions
Acceleration: Put into same electric field → Same kinetic energy
Deflection: Deflected by magnetic field
Detection: Hit detector, strength signal isotope abundance
Data analysis: Produce mass spectrum (peaks for isotopes) showing relative atomic mass & Isotopes abundance
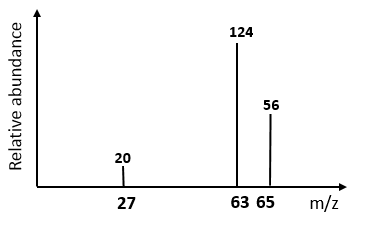
Mass vs Charge
Charge increase → Deflection increase (Direct)
Mass Increase → Deflection decrease (Indirect)
Bohr theory
Energy level are fixed, spherical & orbitual
Occupy from the lowest level (Near nucleus) first
Electromagnetic spectrum (Decreasing Wavelength and Increasing frequency)
Radio waves, microwaves, infrared, visible light, ultraviolet, X rays, Gamma rays
Energy
High frequency = High energy
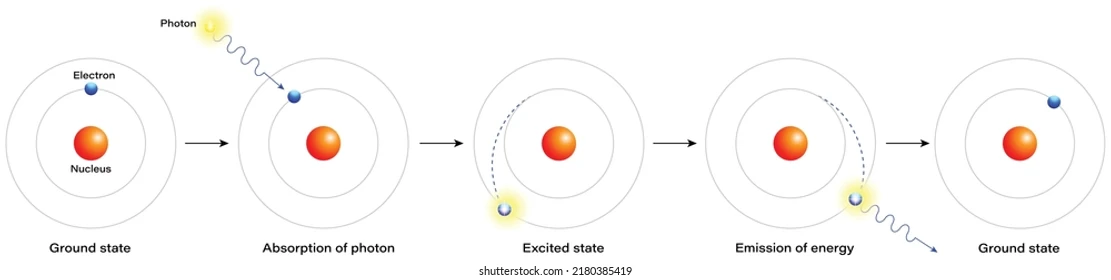
Emission spectra
Ground state: Electron in lowest energy level
Absorption: Photon (energy) absorbed → electron move to high energy level (Excited state - Unstable)
Emission: Electron fall to lower energy level emitting Photon (energy)
Convergence limit
Occur at high energy because…
energy level closer together
Ionisation is the highest (at n infinity, outermost)
Frequency
f = speed of light ( c) / wavelength
Energy of photon
P = Planck Constant (h) (6.626×10^(34)) x f
Energy
E = Avogadro number (n) x P
usually in Joules
J to Kj
Kj = J / 1000
Hydrogen emission spectrum

Energy level
Divided into sublevels (s,p,d,f) depends on orbitual (High % of finding e)
s sublevel
max 2 electron, spherical shaped
p sublevel
max 6 electron (3 orbitual), dumbell shaped
d sublevel
max 10 electron, 5 orbitual
f sublevel
max 14 e, 7 orbitual
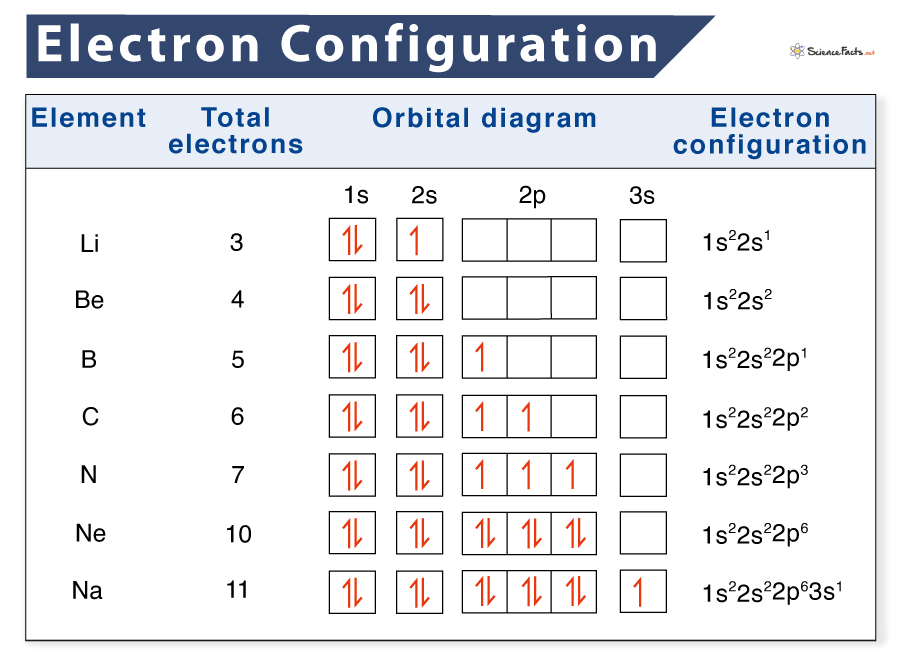
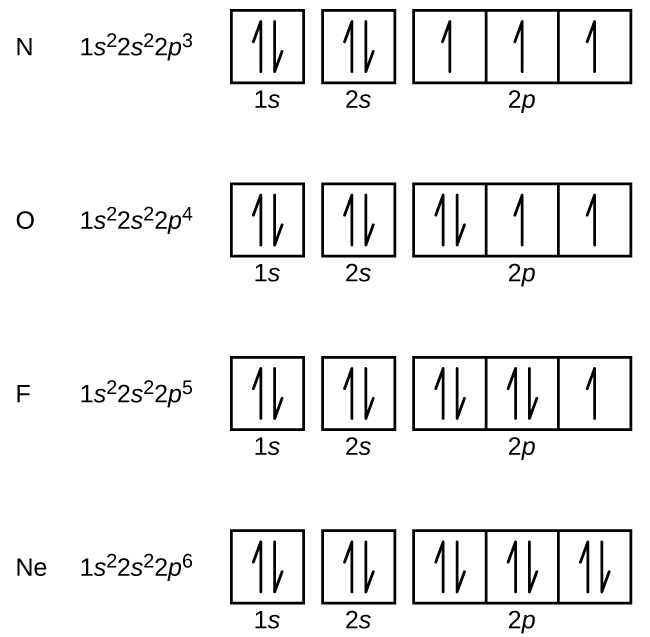
Electron configuration
1s², Energy level, sub level, number of electron
Degenerate orbitual
or bitual within the same sub level of an atom → same energy
d-block element
4s fills first before 3d sublevel
Exception: Cr & Cu
Orbitual diagram
visual reperesentation of electron configuration
Ionisation
The process of removing e from atom in ground state
Repulsion
force that push particle with same charge away (electrons in shells)
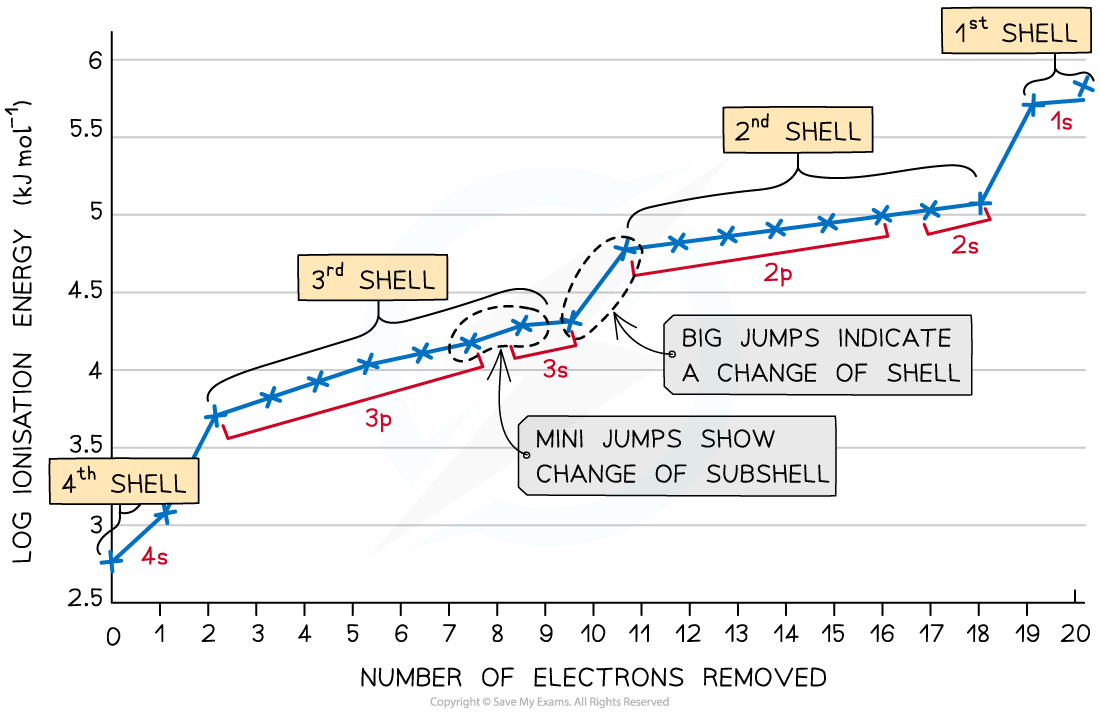
Successive ionisation energies
process of removing electron from atoms, start from higher energy sublevel first
Avogadro constant (n)
6.02 × 10^(23)
1 = 1 moles
Formula unit
simplest ratio of ions in compound
→ convert before finding moles
Moles (n)
n = mass (m) / Molar mass (M)
Number of _ (atom/electron):
N = n x avogadro constant
Percentage composition
convert to mass
convert to mole
equal ratio
Moles (n) in liquid
n = concentration ( c ) x volume (dm³) V
Avogadro law
equal volume of GAS at the same temperature and pressure will have the same number of gas particles
Ideal gases properties
particles are constant (small size), random and move in a straight line motion
weak intermolecular forces between particles
distance between particles > size of particles
Average kinetic energy directly proportional to absolute temperature (Kelvin)
→ Ideal condition: Low pressure and high temperature
Real gas
Deviation from the Ideal gas model due to…
→ High pressure and low temperature
→ slower speed → attraction between particles
→ Liquidified
→ higher molar mass
Standard condition (STP)
273 K (0 C) & 100kPa
Moles in gas
n = V / 22.7 dm^-3 mol-1 (Molar volume)
Constant temperature
Pressure and volume are inversely proportional
P1V1 = P2V2
Constant pressure
Volume of ideal gas directly proportional to absolute temperature (K)
V1/T1 = V2/T2
Constant volume
Pressure of fixed mass directly proportional to absolute temperature (K)
P1/T1 = P2/T2
Combined laws
PV = nRT
P1V1 / T1 = P2V2/T2
Molar mass
M = mRT / PV