Respiratory Acid-Base Balance
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Why is proper pH essential?
• Enzyme reactions are pH sensitive and have and optimum pH range
• Optimum function requires strict regulation of ionic composition of body fluids.
• The concentration of hydrogen ions ([H+]) is reported as pH - determines acidity or alkalinity of body fluids
• Serious deviations outside the normal range can disrupt cell metabolism and body function.
What organs are responsible for regulating pH?
Lungs and kidneys
What is the survival window of pH?
What is the normal pH of arterial blood?
Survival Range: 6.8 - 8.0
Normal: 7.35 - 7.45
Reminder** What happens to CO2 in an aqueous solution?
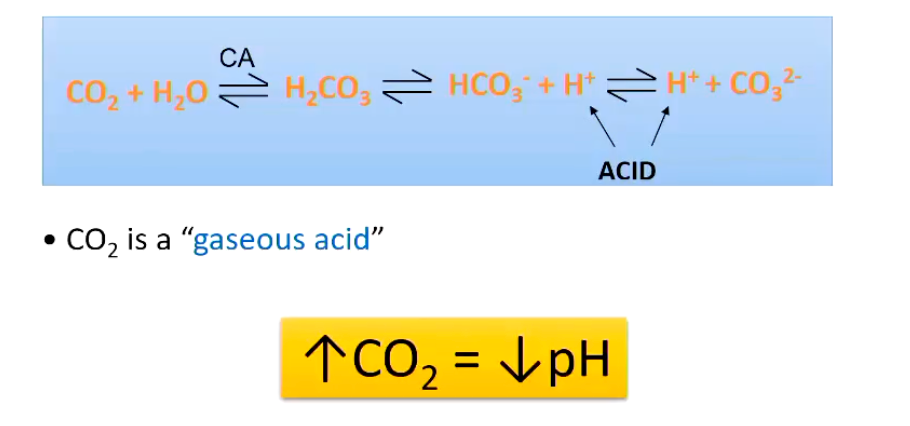
High CO2 leads to a ___ ___.
low pH
What are buffers?
Substances that reversibly bind H+
What does blood pH measure?
Measure of H+ that are not bound to buffers
What is the most important pH buffering system within the body?
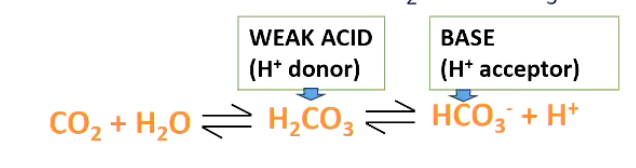
• Most important buffer system in the blood is based on chemical interactions between CO2 and HCO3-
Large amounts of HCO3- in the blood (24 mM/L)
Reactions are reversible, so is dependent on what is on each side of the equation
Describe the other buffering systems used within the body.
Haemoglobin - Imidazole groups bind excess hydrogen ions (Oxyhaemoglobin and Deoxyhaemoglobin)
Intracellular Buffers - H+ can be moved into the cell to buffer
With other molecules
How is acid produced by the body and how is hydrogen ion concentration maintained?
• Cell metabolism continuously produces H+ (acid load)
• Intracellular pH (pHi) must be tightly regulated
• Balance between acid loaders (v pHi) and acid extruders (^ pHi)
• Main buffering system involves bicarbonate (HCO3-)
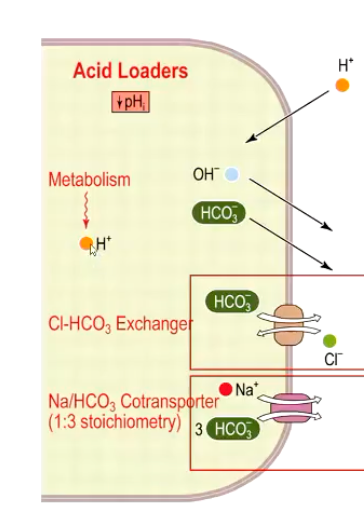
What are the functions of the Acid Loaders?
Lowers intracellular pH (Net effect is increased intracellular H+ and decreased pHi)
Exports bicarbonate → loss of base
Favors HCO3- efflux
What are the functions of the Acid Extruders ?
Raises intracellular pH
Actively pumps H+ out (ATP dependent)
Removes H+ in exchange for Na+
Imports bicarbonate
Promotes HCO3- influx
**The imported HCO3- binds free H+ in the cytoplasm
What provides the first defense against changes in blood pH? What about against large changes in H+ levels?
Blood buffers = first defense
But the lungs and kidneys must ultimately correct the H+ load
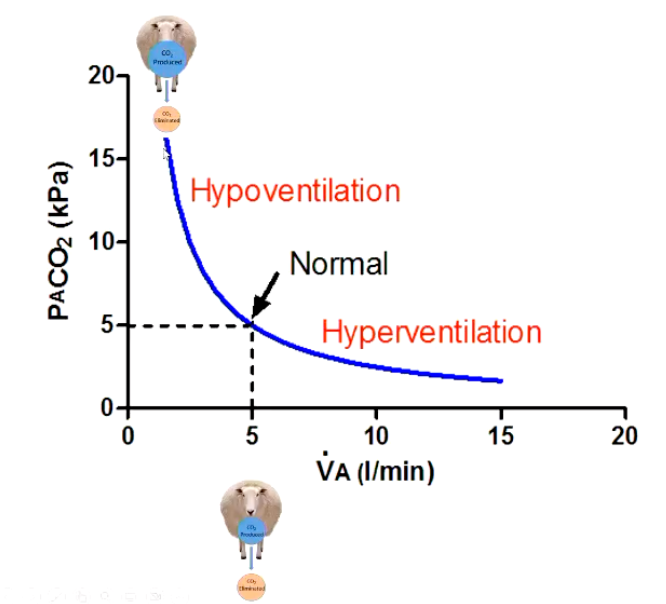
Describe this graph and how ventilation alters the levels of PCO2 and pH.
Hypoventilation
• PACO2 increases
• Leads to hypercapnia
• Acidosis (Decreases pH)
Hyperventilation
• PACO, decreases
• Leads to hypocapnia
• Alkalosis (Increases pH)
How does the kidney alter acid-base balance?
• Kidney is the only route through which H+ ions can be eliminated from the body.
• H+ excretion occurs in the PCT and is coupled to reabsorption of HCO3-
• H+ ions are secreted into the tubular lumen in exchange for Na+
• Na+ and HCO3- are reabsorbed
What is the ratio of HCO3- to PCO2?
For pH 7.4 (Normal) , ratio of 20:1
The 20:1 ratio means bicarbonate concentration is 20 times higher than dissolved CO₂ concentration in normal blood.
When HCO3- is constant, increases in PCO2 cause decreases in pH
Describe the Henderson-Hasselbalch equation and the values constructing a normal pH.
The Henderson-Hasselbalch equation demonstrates that pH is determined by the ratio of bicarbonate (HCO3) to carbon dioxide tension (PCO2), not their absolute values
pH = pK + log [HCO3-]/PCO2
This fundamental relationship means:
Normal ratio maintains pH at 7.40 with PCO2 of 40 mmHg and HCO3 of 24 mEq/L
Changes in this ratio, rather than isolated values, determine the acid-base status
![<p>The Henderson-Hasselbalch equation demonstrates that pH is determined by the ratio of bicarbonate (HCO3) to carbon dioxide tension (PCO2), not their absolute values </p><p class="font-inter text-sm sm:text-base text-slate-600 mb-4"><strong>pH = pK + log [HCO3-]/PCO2</strong></p><p class="font-inter text-sm sm:text-base text-slate-600 mb-4">This fundamental relationship means:</p><ul><li><p><strong>Normal ratio maintains pH at 7.40</strong> with PCO2 of 40 mmHg and HCO3 of 24 mEq/L </p></li><li><p>Changes in this ratio, rather than isolated values, determine the acid-base status</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/323bc7cd-7a5b-456f-b972-bcfe9348392b.png)
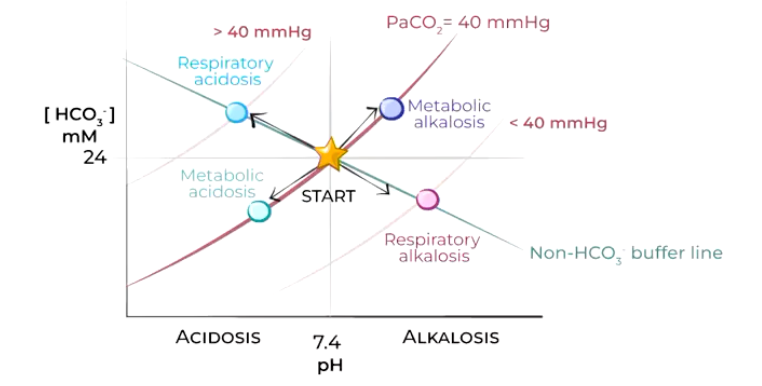
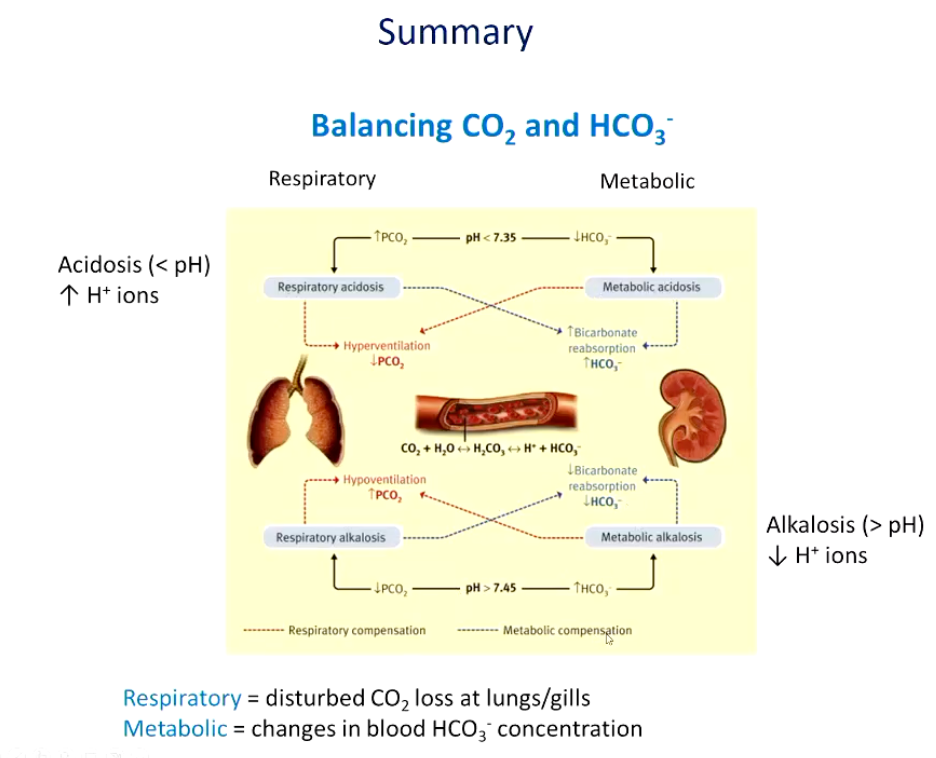
Describe the following acid-base disturbances.
Star = equilibrium between lungs and kidneys (normal pH 7.4, HCO3- 24mmol/L, P,CO, 40mmHg)
• BLUE = Uncompensated respiratory acidosis (hypoventilation) immediate buffering causes small rise in НСО3- (renal compensation will pH back to normal, PCO2 remains elevated)
• PINK = Uncompensated respiratory alkalosis (hyperventilation) immediate buffering causes small fall in HCO3- (reduced renal H* secretion, pH back to normal, PCO2 remains low)
Light blue = Metabolic acidosis reduction in HCO3- concentration (kidneys conserve HCO3, eliminate H* in urine), pH back to normal, PCO2 unaffected- compensation hyperventilation)
Purple = Metabolic alkalosis increase in HCO3- concentration due to loss of Cl ions/excess sodium bicarbonate ingestion (kidneys conserve H+ eliminate HCO3- in alkaline urine), pH back to normal, PCO2 unaffected- compensation hypoventilation-difficult...WHY?)
Overall, if the body fails to keep pH within normal levels, what conditions may result?
• In most diseases, the lungs and kidneys keep pH within tolerable limits.
• Severe disease, homeostatic mechanisms inadequate → life threatening.
Primary problems:
1. Excessive accumulation or elimination of CO2 (RESPIRATORY ABNORMALITIES)
2. Excessive accumulation or elimination of fixed acids or buffer bases (METABOLIC ABNORMALITIES)
How can acid base disturbance alter the distribution of K+ within the body?
• Acidosis causes K+ to move from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of K+ and H+ ions.
• Cl depletion can maintain a metabolic alkalosis (eg after vomiting has stopped) because in absence of Cl, kidney must reabsorb HCO3 with Nat (electroneutrality).
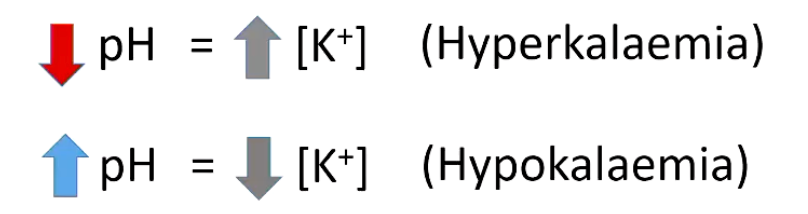
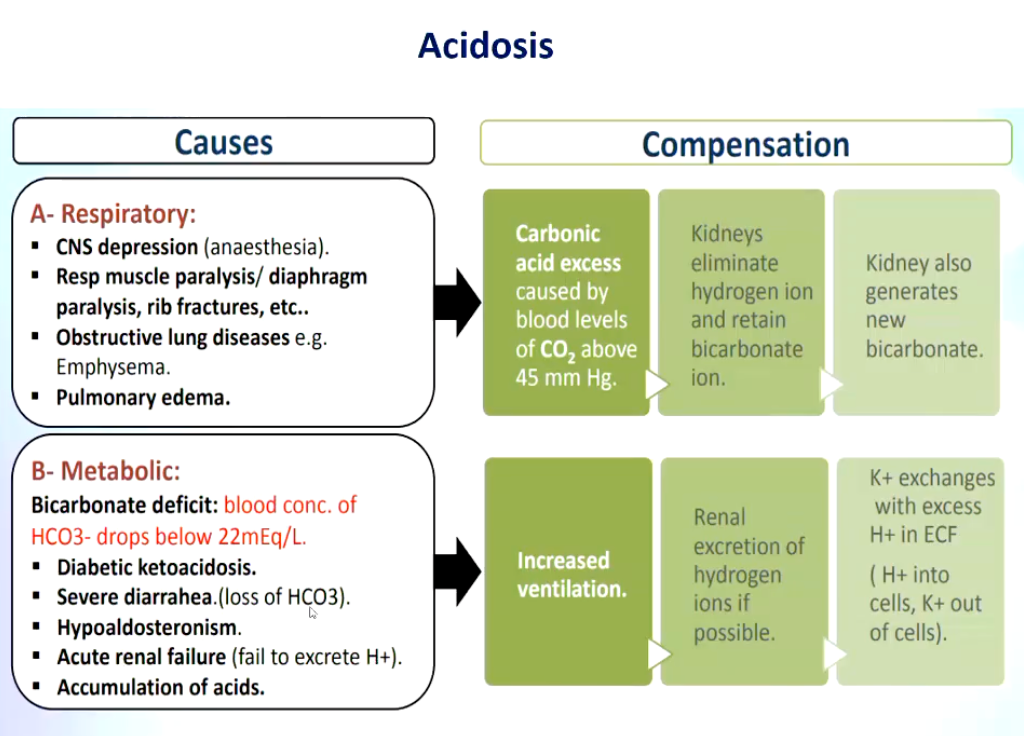
Summarize some respiratory and metabolic causes of acidosis and the subsequent compensation occurring as a result.
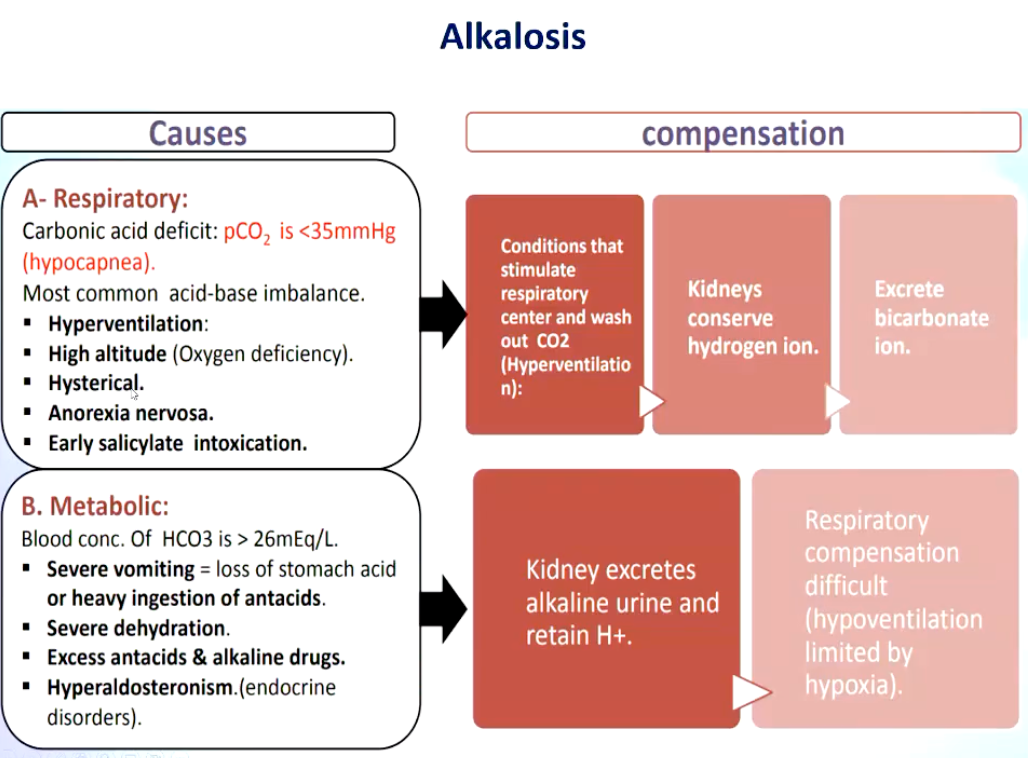
Summarize some respiratory and metabolic causes of alkalosis and the subsequent compensation occurring as a result.
Summarize respiratory and metabolic balance of CO2 and HCO3- levels.