Topic 9 - Structure and Reactivity of Carbonyls
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
What is the shape and hybridisation of a carbonyl?
Flat, sp2 hybridised with a 120 degree bond angle.
What is the Burgi-Dunitz trajectory in carbonyls?
The Burgi-Dunitz trajectory describes the preferred approach of a nucleophile to a carbonyl carbon, typically at a 107-degree angle to the C=O bond. This orientation minimises steric hindrance and facilitates effective orbital overlap during the nucleophilic attack.
Carbonyls react with nucleophiles to form what?
Tetrahedral sp3 hybridised products.

What is nucleophilic addition?
A reaction where a nucleophile attacks a carbonyl carbon, leading to the formation of a tetrahedral intermediate.
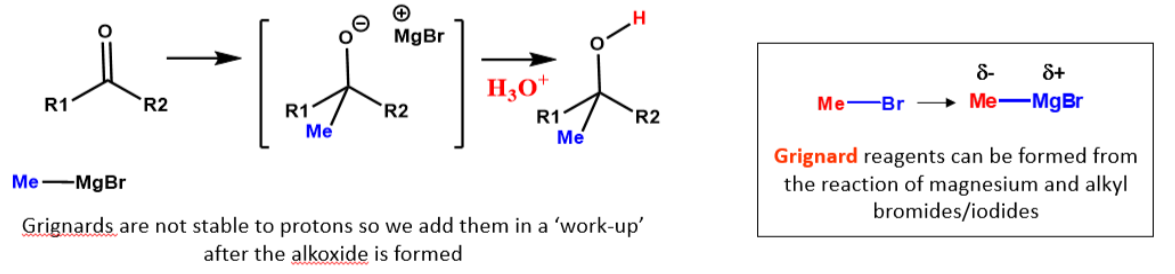
What is Grignard addition?
A reaction where a Grignard reagent, such as methylmagnesium bromide (CH₃MgBr), adds to a carbonyl compound, forming an alcohol after hydrolysis.
The Grignard reagent acts as a nucleophile, attacking the electrophilic carbonyl carbon.
After the addition, aqueous acid (H₃O⁺) is used to protonate the oxygen, yielding the final alcohol product.