Molecular geometry and shape chemistry
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms

linear
number of bonds around a central atom: 2
number of lone pairs around a central atom: 0
number of charge clouds: 2
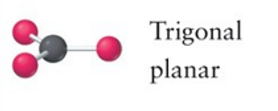
Trigonal planar
3, 0, 3

bent
2, 1, 3
120 degrees
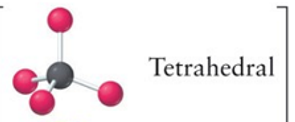
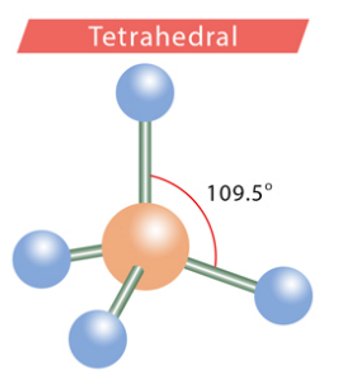
tetrahedral
4, 0, 4
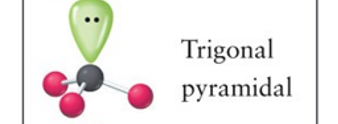
Trigonal pyramidal
3, 1, 4
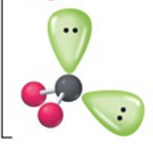
bent
2, 2, 4
109.5 degrees
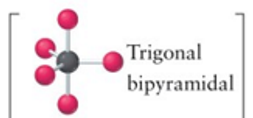
Trigonal bipyramidal
5, 0, 5
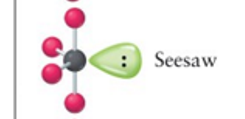
seesaw
4, 1, 5
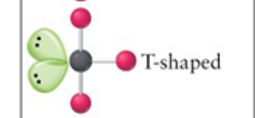
T-shaped
3, 2, 5
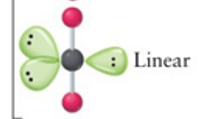
linear
2, 3, 5
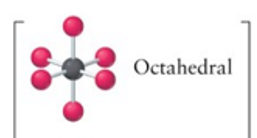
octahedral
6, 0, 6
Square pyramidal
5, 1, 6
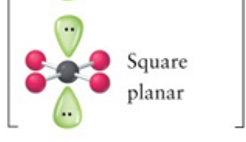
Square planar
4, 2, 6
molecular geometry
describes the three-dimensional shape of just the atoms
electron geometry
describes the shape of all electron groups
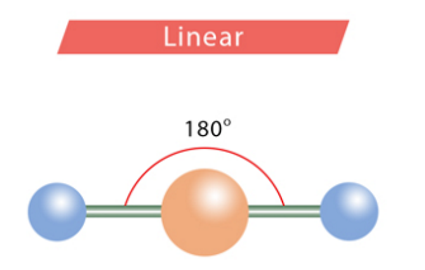
Bond angles: linear
180 degrees
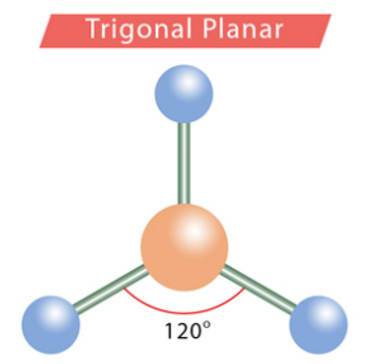
bond angles: trigonal planar
120 degrees
bond angles: tetrahedral
109.5 degrees
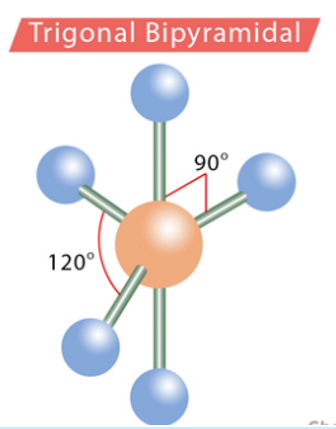
trigonal bipyramidal
120 degrees, 90 degrees
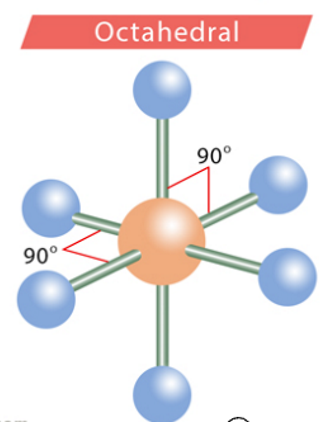
octahedral
90 degrees
sp
electron group: 2
geometry: linear
angle:180
sp²
electron group: 3
geometry: trigonal planar, bent
angle: 120
sp³
electron group: 4
geometry: trigonal bipyramidal, bent, tetrahedral
angle: 109.5
sp³d
electron group: 5
geometry: trigonal bipyramidal, seesaw, T-shaped
angle: 90, 120, 180
sp³d²
electron group: 6
geometry: octahedral, square pyramidal, square planar
angle: 90, 180