Nuclear Chemistry and Half-Lives Review
1/11
Earn XP
Description and Tags
A set of flashcards covering key concepts related to atomic structure, isotopes, and radioactive half-life.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
Atomic Number
The number of protons in the nucleus of an atom, which determines the element's identity.
Mass Number
The total number of protons and neutrons in an atom's nucleus.
Isotopes
Atoms of the same element that have the same atomic number but different mass numbers due to varying numbers of neutrons.
Half-Life
The time required for half of the radioactive material in a sample to decay.
Decay
The process by which an unstable atomic nucleus loses energy by radiation.
Radioactive
A term describing substances that decay and emit radiation.
Graph of Half-Life
A visual representation that shows the amount of radioactive material remaining over time.
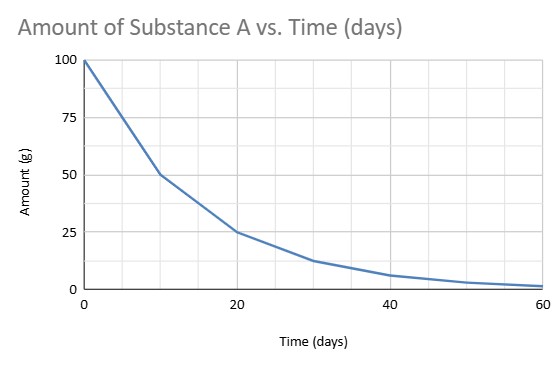
What is the half life of this material?
10 Days
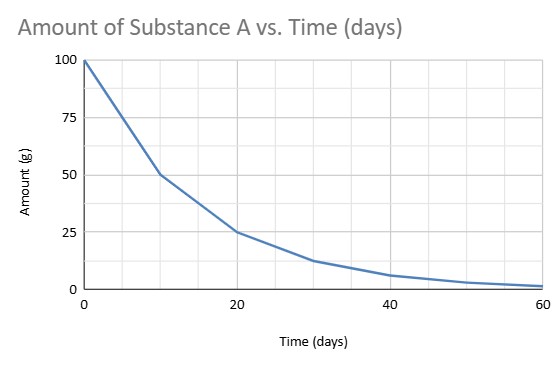
If 50% of the material remains of a sample, how long did that decay for?
10 Days
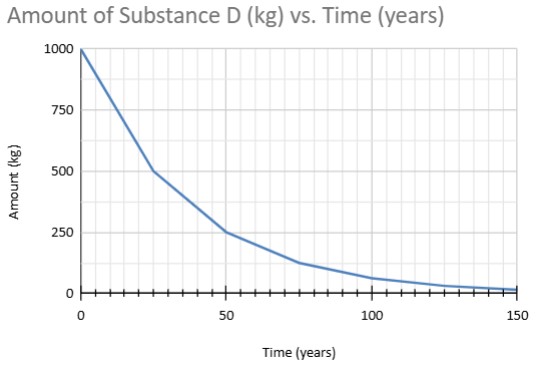
What is the half life of this material?
15 years
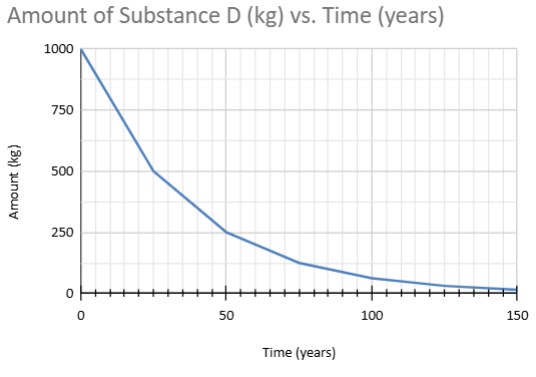
If 25% of the sample remains, how many lives have occured?
2 lives
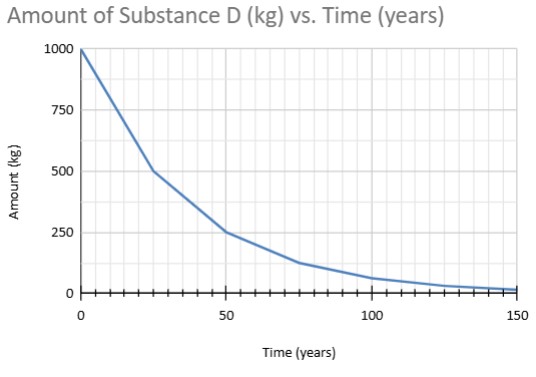
If 12.5% of the sample remains, how long did it take?
45 years