Amino acids, Proteins and DNA
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
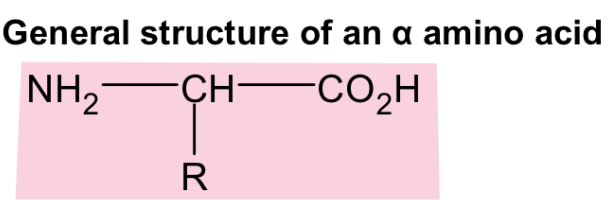
general structure of amino acid
optical activity of amino acids
all amino acids, except glycine, display optical isomerism bc they are chiral
they can rotate plane polarised light
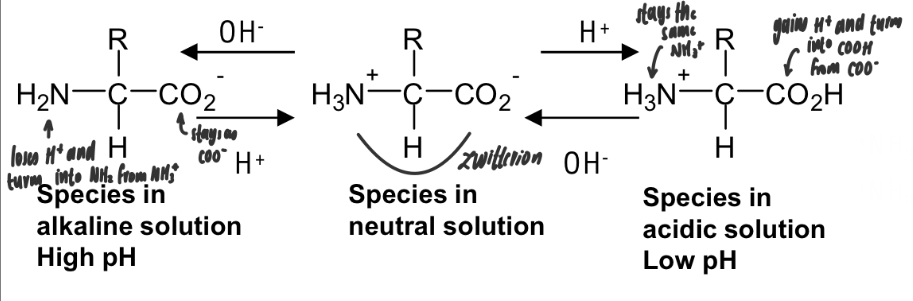
zwitterions
molecules with both positive and negative ions
only exist at the amino acids isoelectric point (the pH at which the overall charge is zero)
the ionic interaction between zwitterions explains the relatively high melting points of amino acids
as opposed to the weaker hydrogen bonding that would occur in the no charge form
acidity & basicity of amino acids
the amine group is basic and the carboxylic acid group is acidic
so amino acids are amphoteric
when in an alkaline solution with high pH
at high pH, the NH3+ is likely to lose a H+ ion, forming NH2
when in an acidic solution with low pH
at low pH, the COO- is likely to accept a H+ ion, forming COOH
amino acids act as weak buffers
will only gradually change the pH if small amounts of acid or alkali are added
dipeptides
combination of two amino acids with one amide (peptide) link
must have CONH link !!
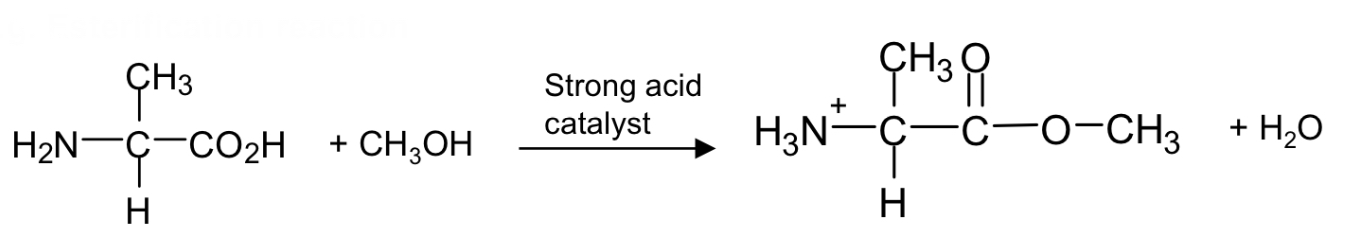
esterification reaction of amino acids with alcohol
shows the reaction of carboxylic acid with an alcohol (e.g. methanol) in the presence of a strong acid catalyst (H+ e.g. sulfuric acid)
forms an ester and water (byproduct)
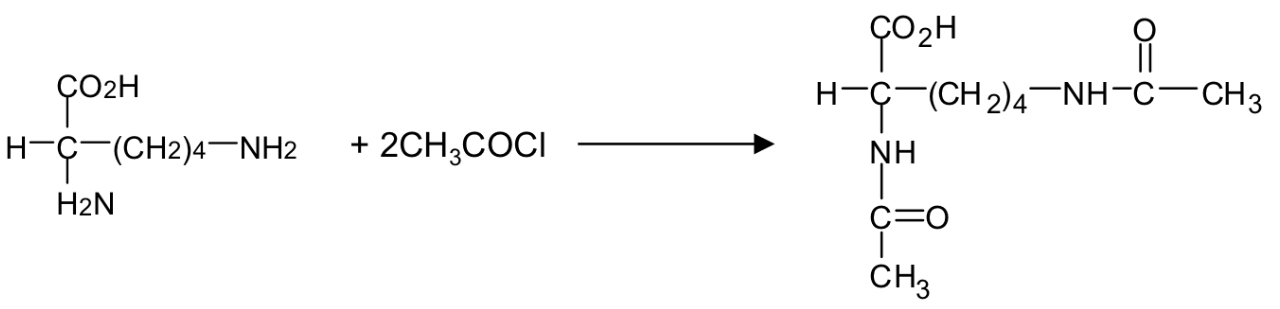
esterification reaction of amino acids with acyl chloride
shows the reaction of carboxylic acid with an acyl chloride
basically the Cl gets removed alongside one H, and the NH then bonds with COCH3
what happens when an amino acid reacts with an excess of a haloalkane
excess haloalkane (e.g. bromomethane) + amine (NH2) in amino acid results in a successive nucleophilic substitution reaction
(rmbr that NH2 is a nucleophile!)
results in the formation of a quaternary ammonium ion
draw the structure of the species formed when glycine reacts with an excess of bromoethane
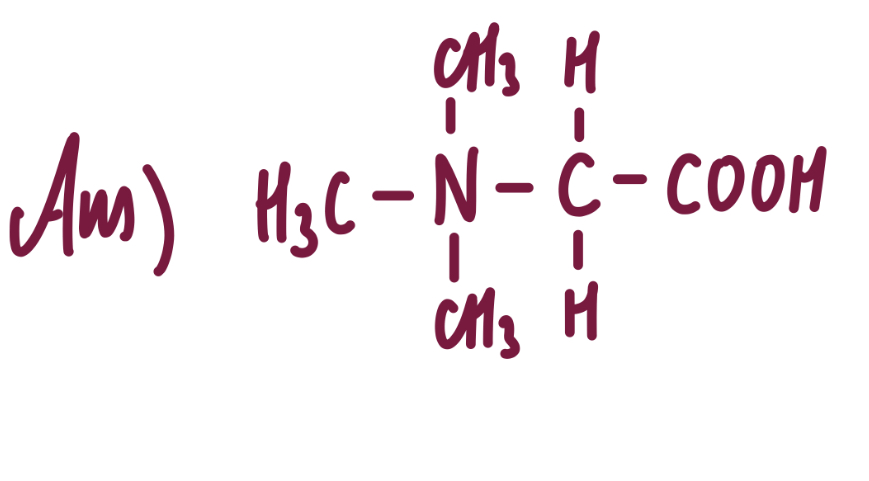
hydrolysis of dipeptides/proteins
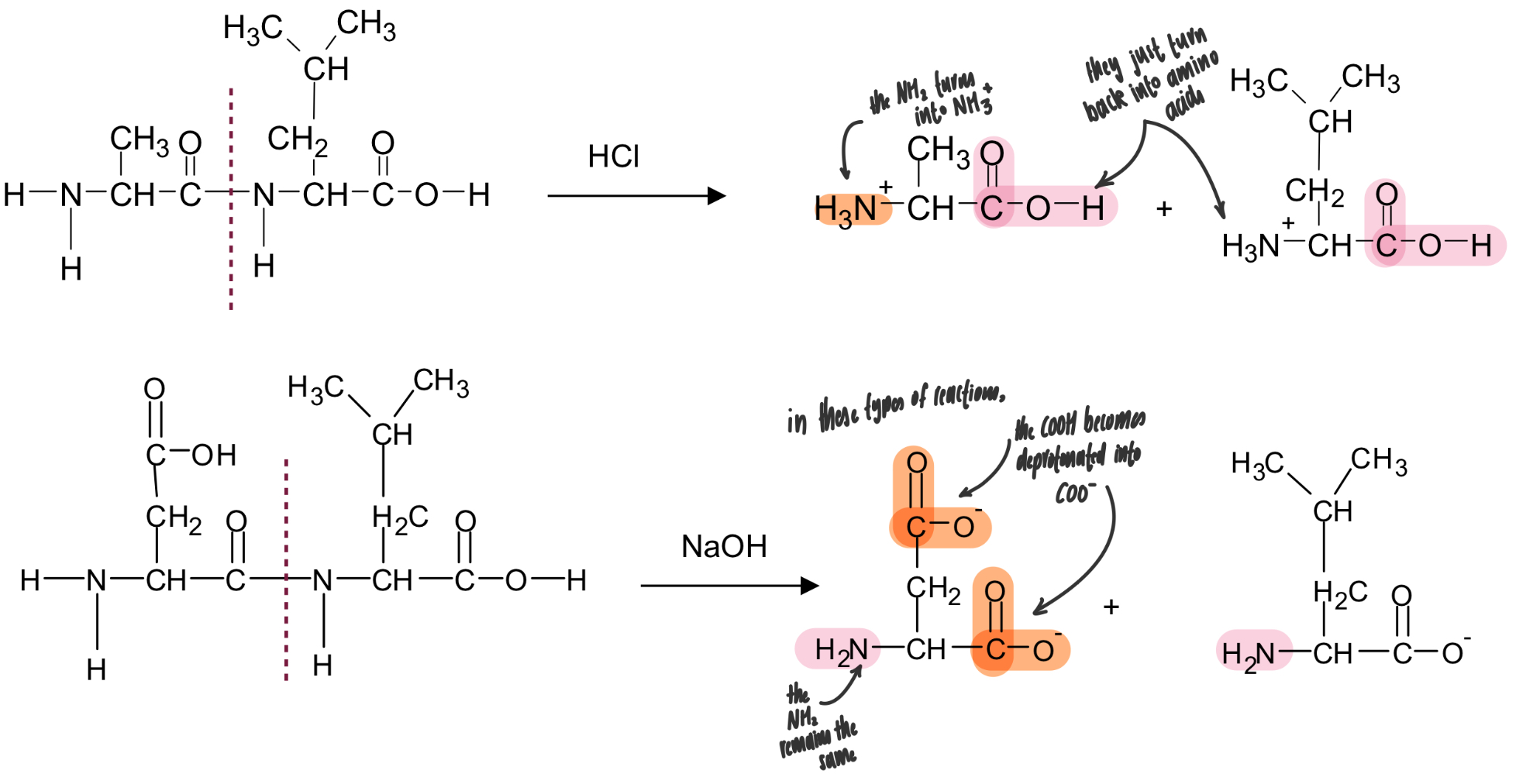
if proteins are heated with concentrated hydrochloric acid or concentrated strong alkalis they can be hydrolysed and split back into their constituent amino acids
the composition of the protein molecule may then be deduced using TLC chromatography
TLC chromatography steps
Wearing gloves, draw a pencil line 1 cm above the bottom of a TLC plate
Use a capillary tube to add a tiny drop of each solution to a different spot on TLC plate
allow the plate to air dry
Add solvent to a chamber with a lid
ensure solvent is below pencil line
When the level of the solvent reaches about 1 cm from the top of the plate, remove the plate and mark the solvent level with a pencil
Allow the plate to dry in the fume cupboard.
Spray paper with ninhydrin
Draw around them lightly in pencil.
Calculate the Rf values of the observed spot
importance of wearing gloves
to prevent contamination form the hands to the plate
importance of pencil line
so it will not dissolve in the solvent
importance of tiny drop
too big of a drop will cause different spots to merge
importance of the depth of the solvent
if the solvent is too deep it will dissolve the sample spots/mixture from the plate
importance of the lid
to prevent evaporation of toxic solvent
importance of drying in a fume cupboard
important as the solvent is toxic
is allowing the solvent to rise to the top of the plate essential?
not essential
will get more accurate results if the solvent is allowed to rise to near the top of the plate
but the Rf value can be calculated if the solvent front does not reach the top of the plate
why is ninhydrin sprayed on the amino acids?
bc amino acids are transparent and cannot be seen
can also shine UV light to see the position of the spots
outline the steps needed to locate the positions of the amino acids on the TLC plate and to determine their Rf values
spray with developing agent or use UV
measure distance from initial pencil line to the spots (x)
measure distance from the initial pencil line to the solvent front line (y)
Rf value = x/y
how to identify the amino acid using the Rf value
measure how far each spot travels relative to the solvent front and calculate the Rf value
each amino acid has its own Rf value
compare Rf values to those for known substances
explain why different amino acids has different Rf values
amino acids have different polarities
therefore they have different retention on the stationary phase and different solubility in the developing agent
suggest why lysine leaves the column after alanine
lysine has a more positive charge
so it has a greater affinity to the stationary phase
so it adheres better to the stationary phase
proteins definition
polymers made from combinations of amino acids formed by condensation reactions
linked by peptide bonds
primary structure
the sequence of amino acids joined together via condensation reaction, forming peptide bonds
secondary structure
alpha-helix & beta-pleated sheets
held in place by H-bonds between the H in N-H group and the O in C=O group
tertiary structure
folding of secondary structure to form 3D shapes
held in place by interactions between R groups (e.g. H-bonds, ionic bonds and disulfide bridges)
stereospecific active site
If the substrate is chiral then it’s likely that only one enantiomer will have the correct stereochemistry to fit in the active site of the enzyme
so only one isomer will be catalysed.
Drugs as Enzyme Inhibitors
Many drugs act as an enzyme inhibitor by blocking the active site
The inhibitor will often bind to the active site strongly so stopping the substrate attaching to the enzyme
Some inhibitors can also attach elsewhere on the enzyme but in doing so can change the shape of the active site, which also stops its effectiveness
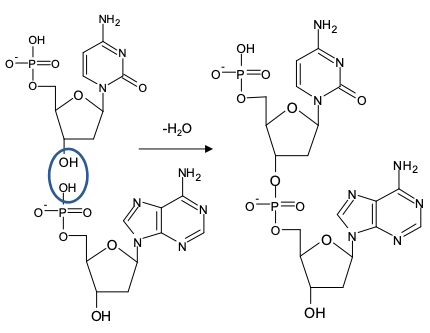
how sugar-phosphate chain is formed
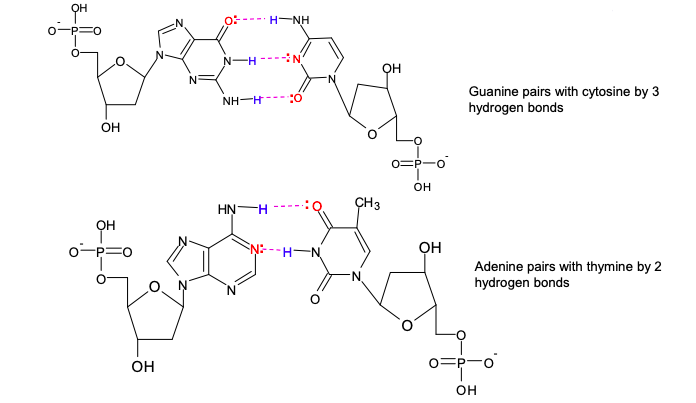
explain how cytosine forms a base pair with guanine
the top N-H on cytosine forms a H-bond to the l.p. of electrons on the oxygen on guanine
the l.p. of electrons on nitrogen in cytosine H-bonds to the H-N on guanine
a l.p. of electrons on the oxygen on cytosine bonds to the lower H-N on guanine
why can no other base pairs in DNA beside A-T and C-G form?
no other base pairing can be formed because:
the partially charged atoms would be too close to each other and repel
the bases would not get close enough to each other for H-bond to form
complementary base pairing
cisplatin
Cisplatin prevents DNA replication in cancer cells by a ligand replacement reaction with DNA
coordinate bond is formed between platinum and a nitrogen atom on guanine
Cisplatin can also prevent the replication of healthy cells by attaching to healthy DNA in normal cells
why does cisplatin work as an anticancer drug but transplatin doesn’t?
The cisplatin version only works as two chloride ions are displaced and the molecule joins on to the DNA
In doing this it stops the replication of cancerous cells
side effects of cisplatin and how it can be minimised
use of cisplatin may lead to unwanted side effects like hair loss.
unwanted side effects can be minimised by giving cisplatin in small doses/amounts and targeting the application to the tumour