CHEM 108: Chpt 14 - Carbohydrates Structure and Function
1/55
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
56 Terms
What is the general formula of a carbohydrate?
(CH2O)n
What is the suffix for sugar?
-ose
Monosaccharides
Single Sugar Unit
cannot be hydrolyzed
Disaccharides
Two Sugar Unit
Reacts with water to produce two monosaccharides
Polysaccharides
Many sugar units
Reacts with water to produce many monosaccharides
Biological polymers
What do monosaccharides exist as?
Stereoisomers
What is a stereoisomers?
1. Same molecular formula
2. Same connectivity
3. Different 3D orientation of atoms
When do stereoisomers exist?
When a molecule is chiral (NOT IDENTICAL)
What is Chirality?
The symmetry of objects
What is a chiral molecule?
When objects and molecules are non-identical
What makes two molecules non-superimposable( not identical)?
When they cannot be placed on top of each other to match perfectly in 3D space, even after rotation.
What is another term for chirality?
Handedness
What is an example of chirality?
Hands. When you look at hands, they mirror each other, but when you place one on top of the other, they don't line up.
What is a Chiral Carbon?
A tetrahedral carbon that is singly bonded to 4 different atoms.
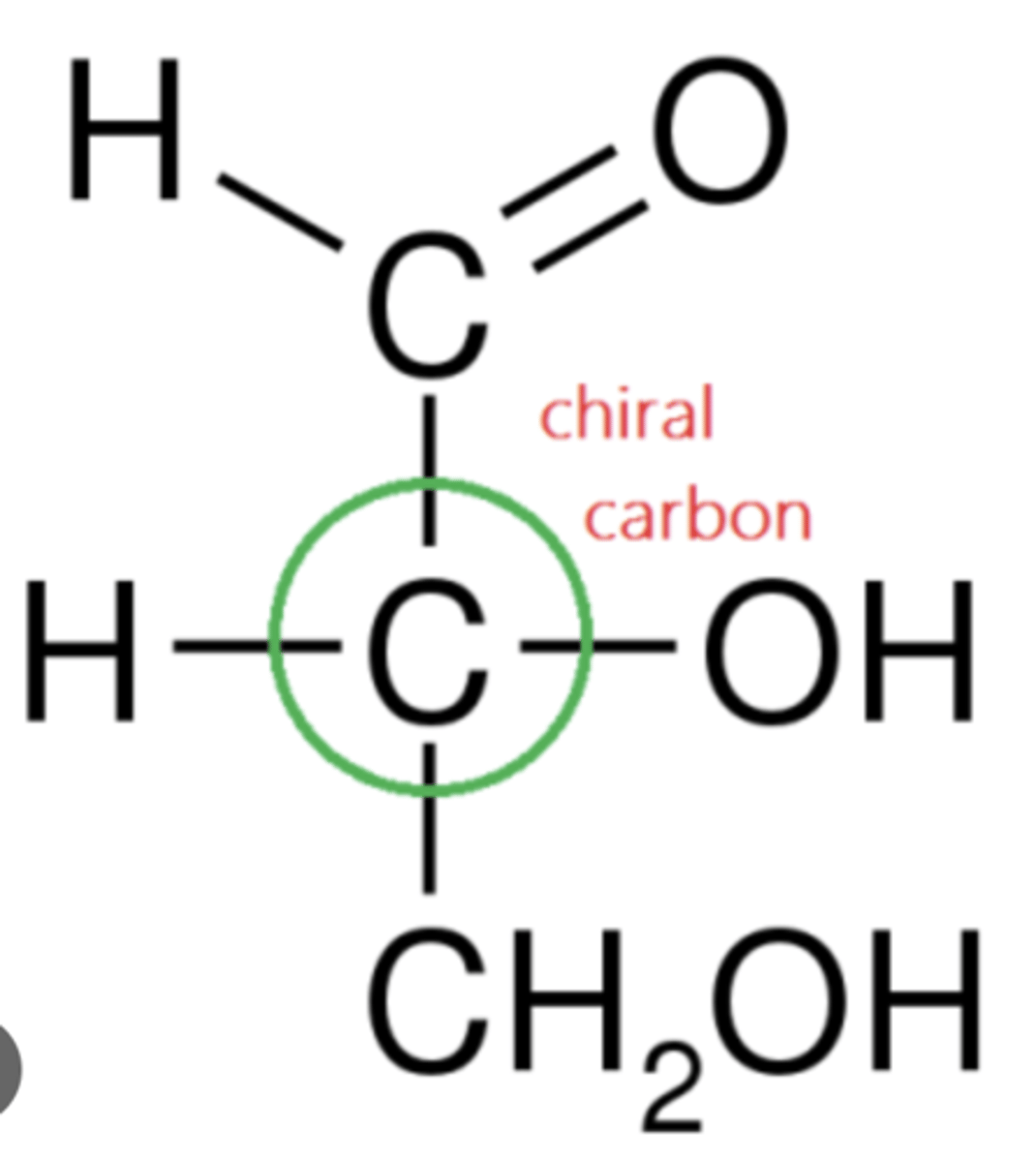
Why are chiral carbons asymmetrical?
It has four different groups attached → so its 3D shape isn’t balanced/symmetrical.
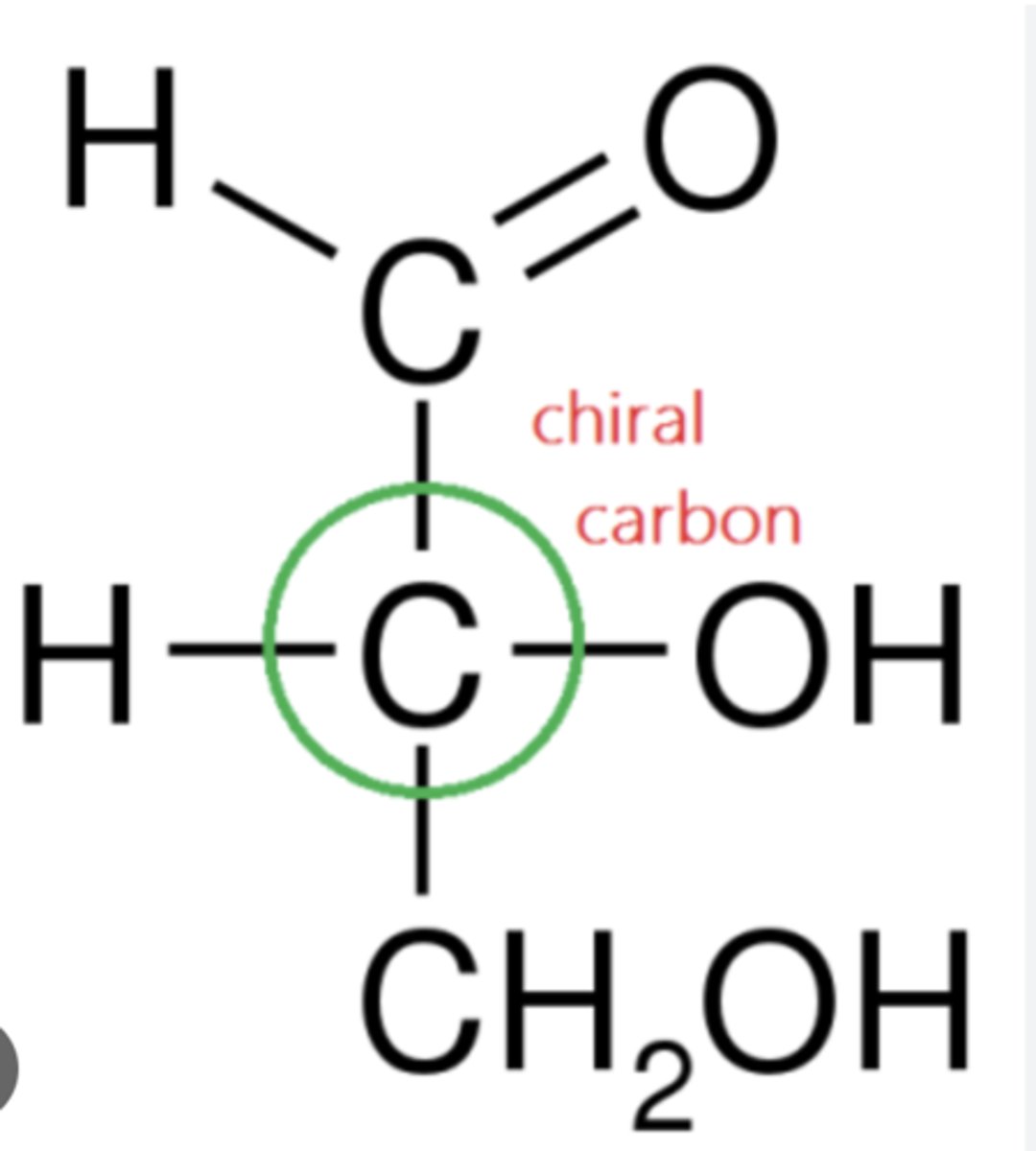
Define Achiral:
Molecules and objects that are identical to their mirror images
What are Enantiomers?
A set of two nonsuperimposable (non-matching) mirror images
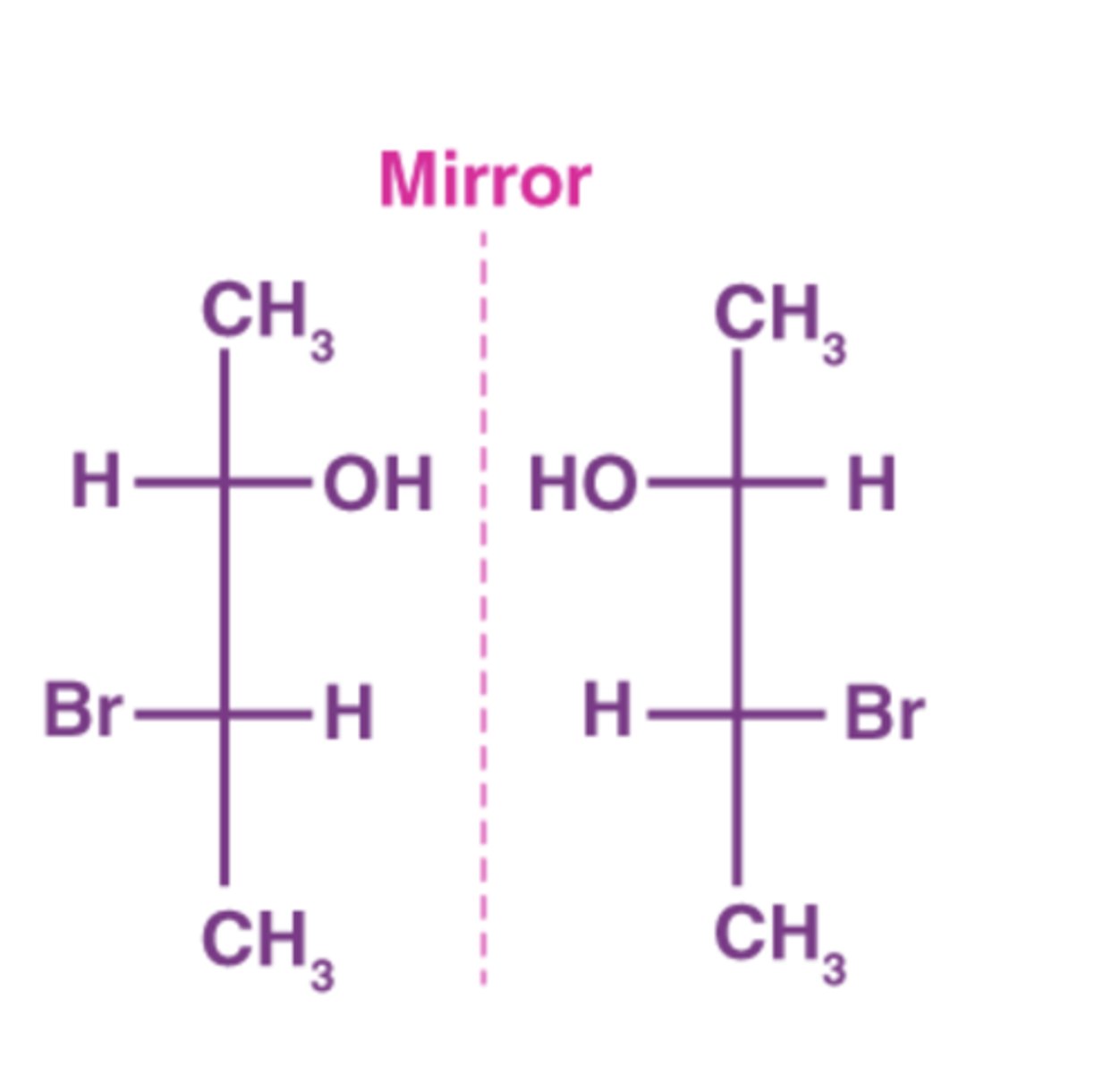
What must enantiomers contain?
At least 1 chiral carbon
What structural feature causes a molecule to have an enantiomers?
A chiral carbon → makes the molecule asymmetrical → which allows for two non-superimposable mirror images → those mirror images are enantiomers.
How many mirror-image forms does a molecule with one chiral carbon have?
Two
If a molecule has at least one chiral carbon, what can we say about the whole molecule?
The entire molecule is considered chiral
What type of projection is commonly used in biochemistry to draw sugars?
Fischer projections
What do vertical and horizontal lines represent in a Fischer Projection?
Fischer Projections use vertical and horizontal lines to show the 3D orientation of bonds.
What does an intersection in a Fischer Projection indicate?
A chiral carbon with four different groups attached.
When drawing enantiomers on Fischer Projections, what happens to all the horizontal atoms?
They flip sides
What is the D vs L notation based on?
The location of the OH on the bottom chiral carbon only
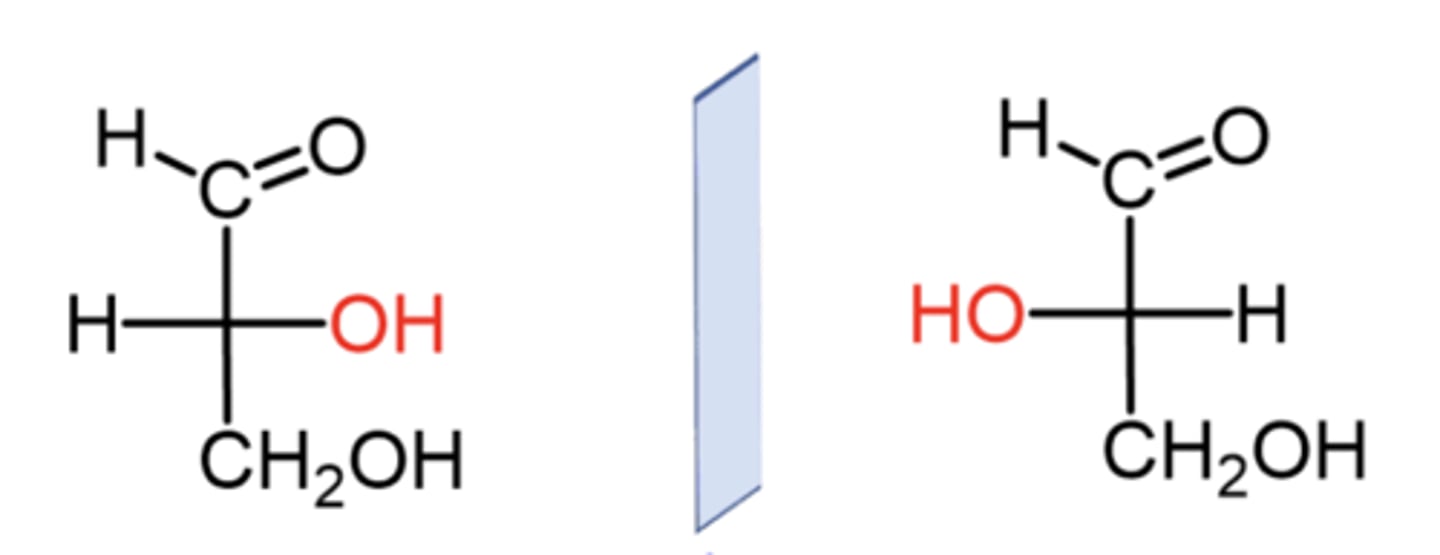
If the OH on the last (bottom) chiral carbon is on the right, which enantiomer is it?
The D enantiomer
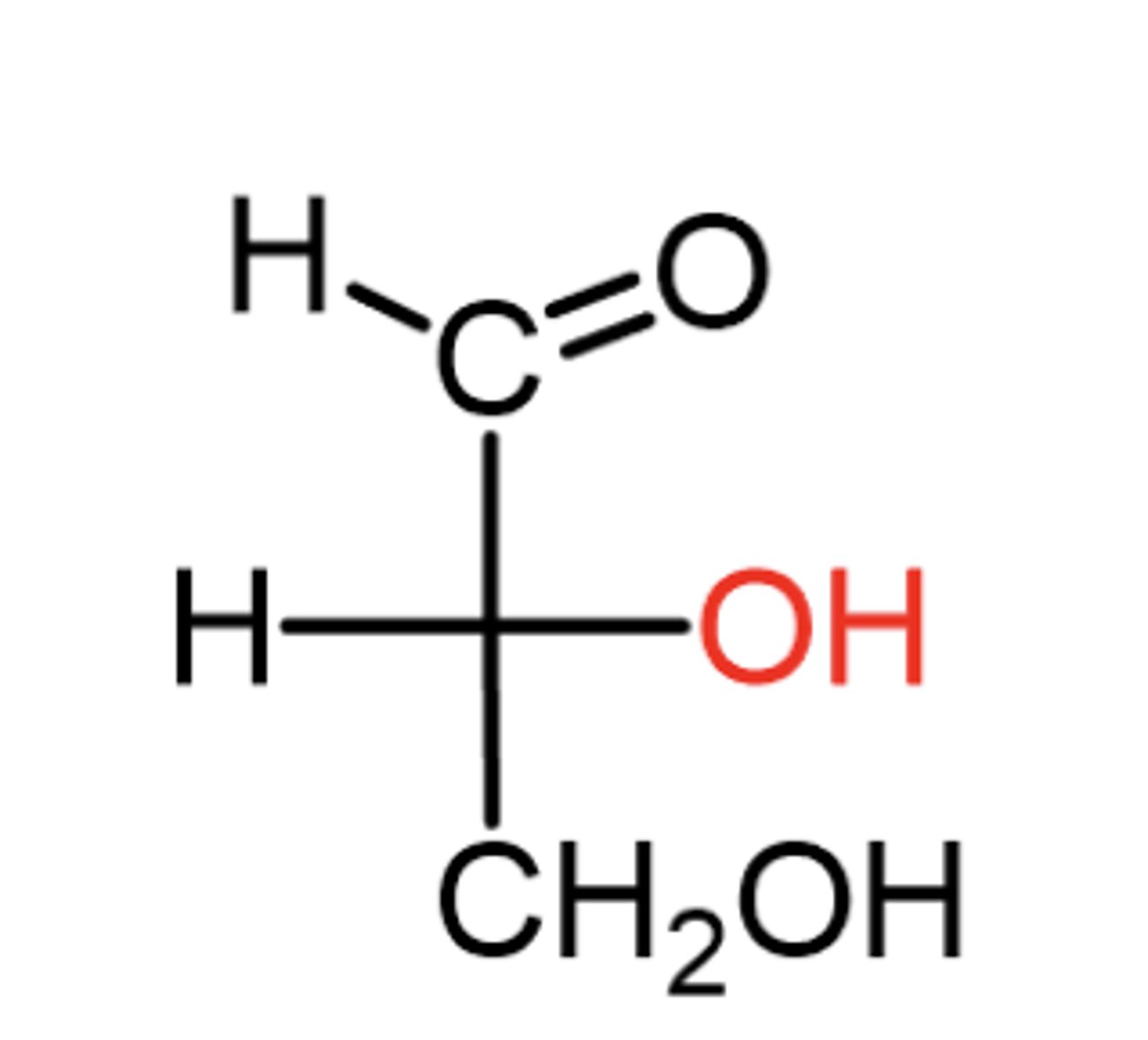
If the OH on the last (bottom) chiral carbon is on the left, which enantiomer is it?
The L enantiomer
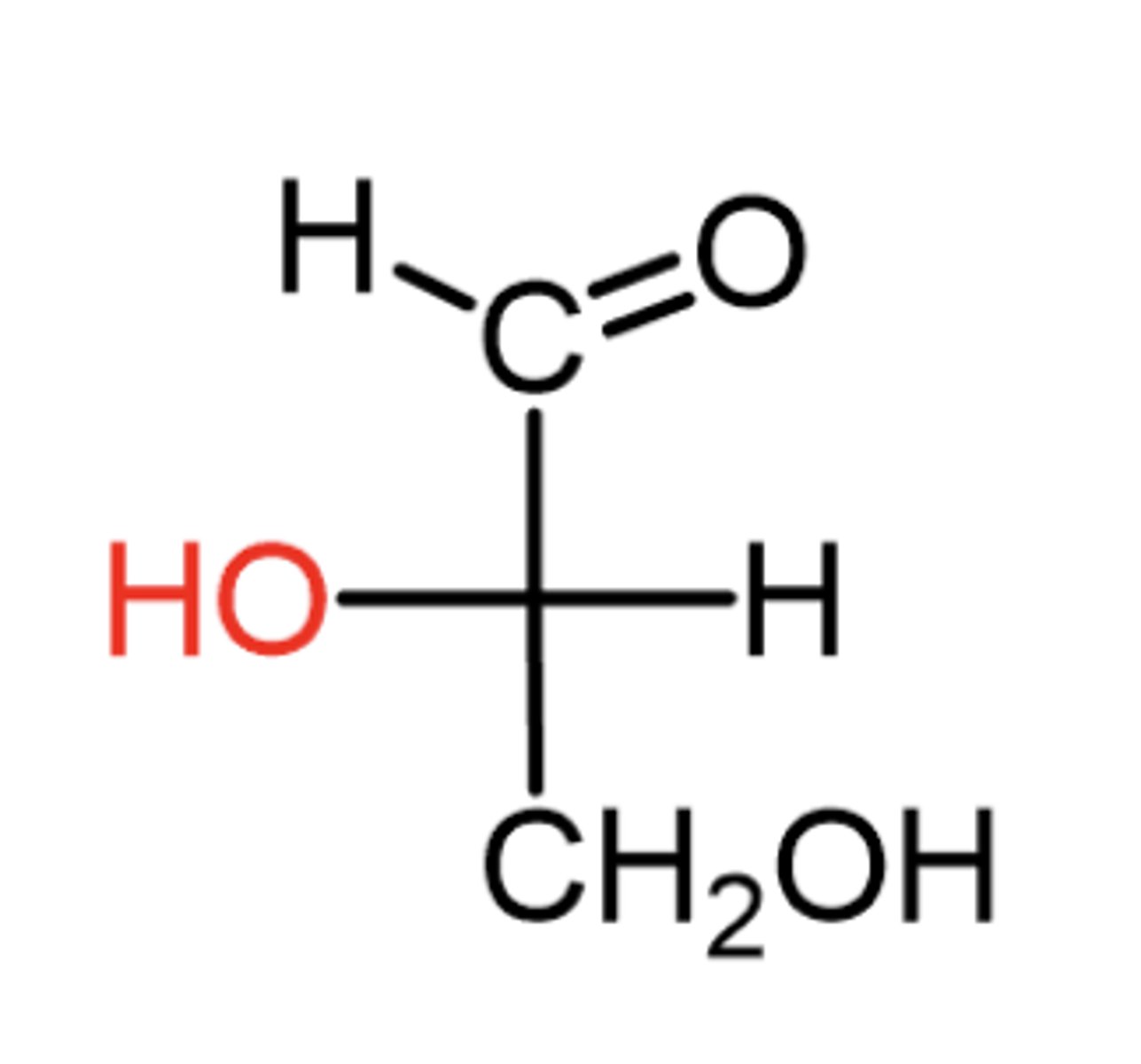
What properties are identical in a pair of enantiomers?
melting point, boiling point, solubility
Why do enantiomers behave differently in a chiral environment?
because they bind differently to chiral molecules like enzymes and receptors
What is a chiral environment?
any environment that is not symmetrical
What is a racemic mixture?
a 50:50 mix of enantiomers
What are the 3 main structural features used to classify monosaccharides?
1. How many carbons the sugar has
(triose, tetrose, pentose, hexose)
2. What functional group it contains
Aldehyde → aldose
Ketone → ketose
3. The arrangement of the chiral carbons
(D or L configuration based on the highest-numbered chiral carbon)
What is name for a 3 carbon chain?
Triose
What is the name for a 4 carbon chain?
Tetrose
What is the name for 5 carbon chain?
Pentose
What is the name for a six carbon chain?
Hexose
How do D-glucose and D-galactose differ?
They differ by the direction the OH points at one of their chiral centers.
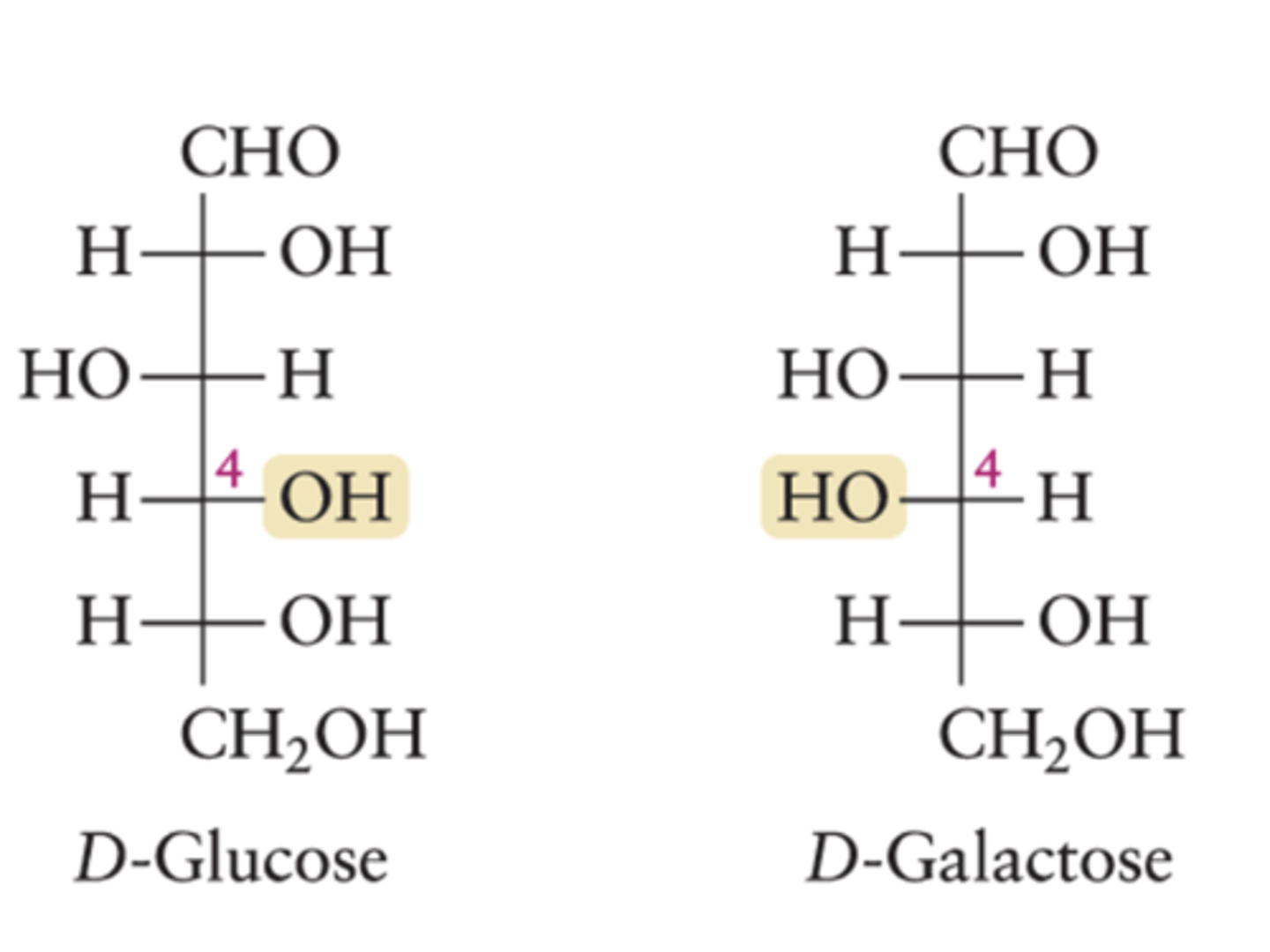
What is implied when the D notation is left out?
That all sugars are in the D configuration
What type of ring forms do monosaccharides?
Very stable five- or six-membered rings with an oxygen atom in the ring
What is a pyranose ring?
A six-atom ring with an oxygen atom in the ring.
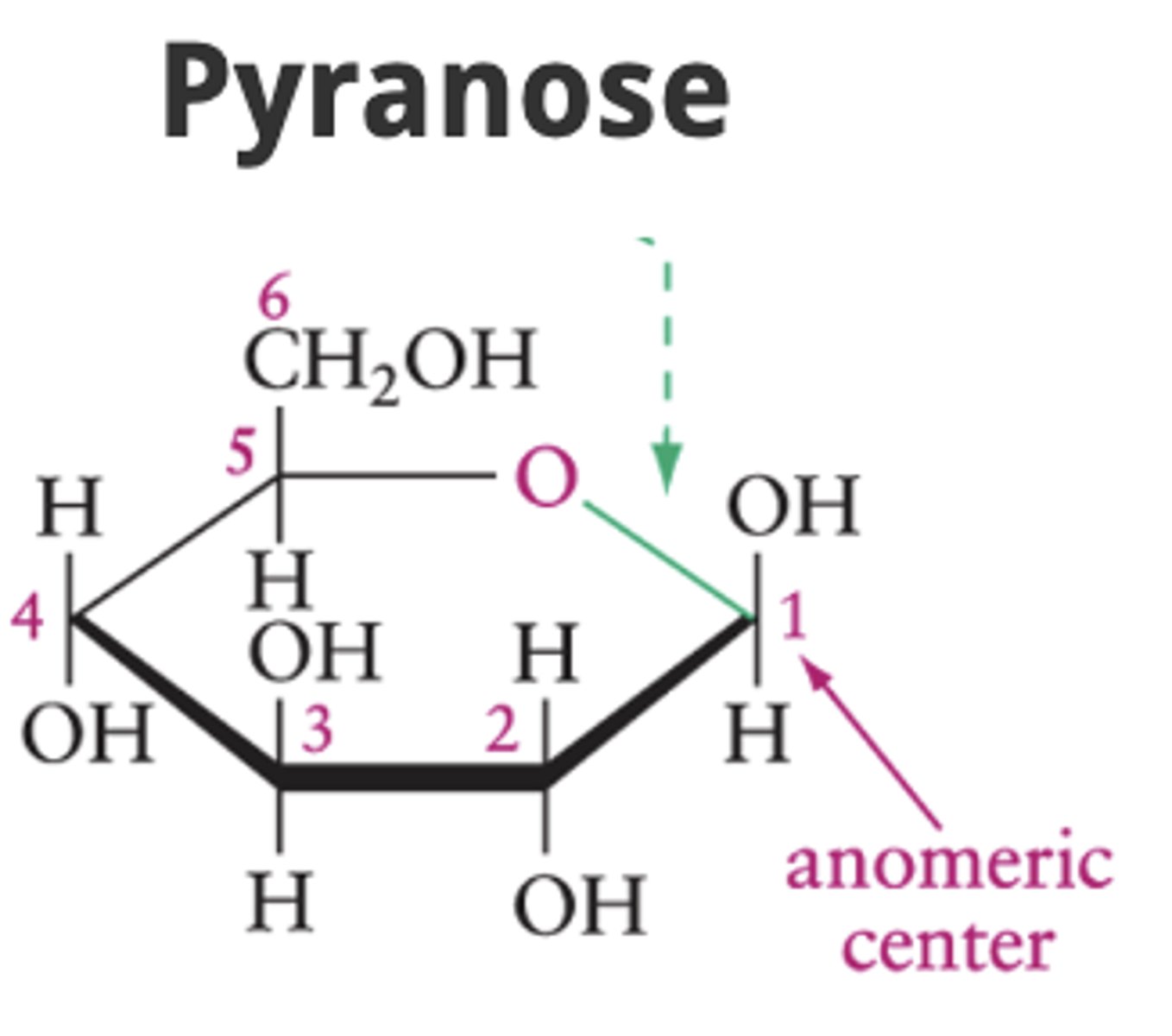
What is a furanose ring?
A five-atom ring with an oxygen atom in the ring.
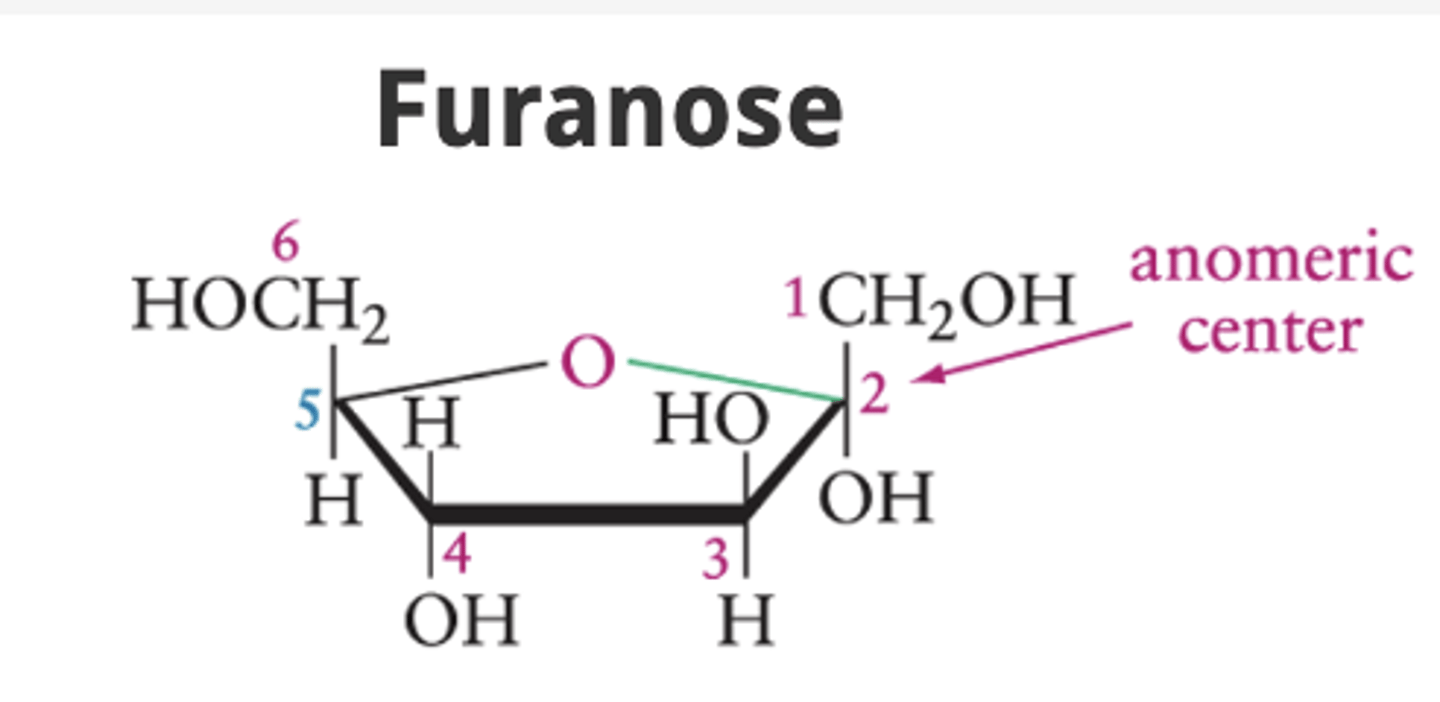
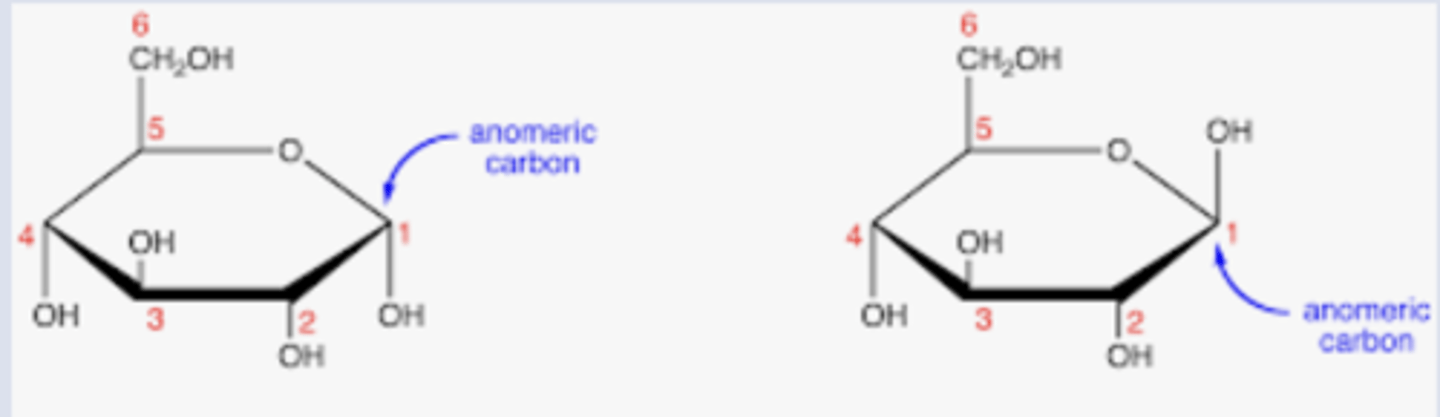
What distinguishes the α- and β-anomers?
The position of the OH at the anomeric carbon
In an α-anomer, where is the OH at the anomeric carbon located?
Below the ring (pointing down)
In a β-anomer, where is the OH at the anomeric carbon located?
Above the ring (pointing up)
Anomeric center (carbon)
the "special carbon" that flips up or down when a sugar closes into a ring.
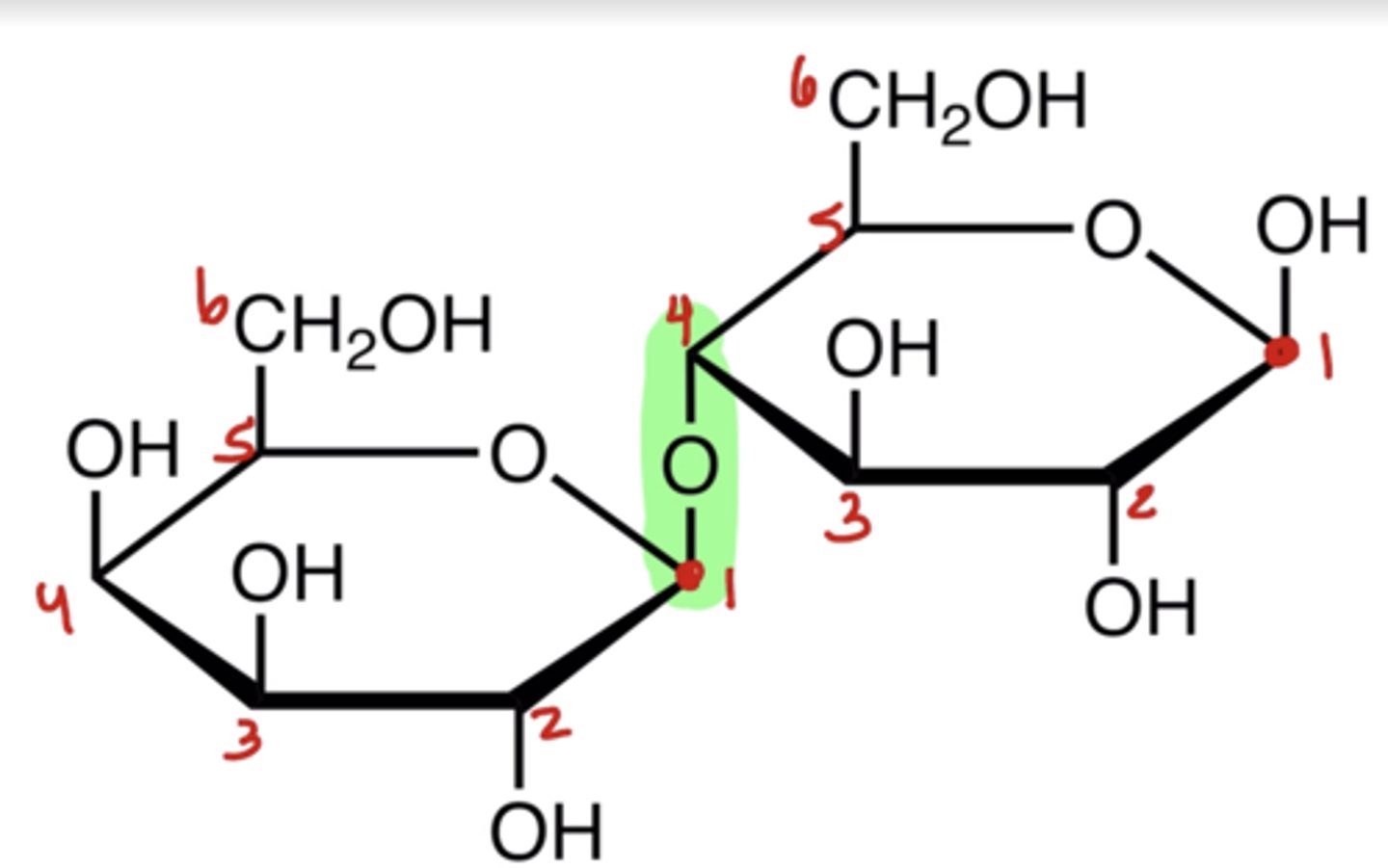
Complex carbohydrates are derived from what?
two or more monosaccharides joined by glycosidic bonds
What types of carbohydrates are included in complex carbohydrates?
Disaccharides, oligosaccharides, and polysaccharides
When maltose is hydrolyzed with water, what forms?
Two molecules of glucose
When sucrose is hydrolyzed with water, what forms?
A molecule of glucose and a molecule of fructose
When naming a glycosidic linkage, what steps do you follow?
1. Number each sugar separately and identify carbon #1 (the anomeric carbon) on each ring.
2. Identify which carbons are linked between the two sugars (e.g 1 &4)
3. Determine whether the anomeric carbon forming the bond is α or β.
What are examples of polysaccharides?
starch, glycogen, and cellulose
What is starch made of, and what happens to it during digestion?
1. Starch is composed of: 20% amylose and 80% amylopectin
2. when digested, it is hydrolyzed into glucose & any excess glucose gets converted to glycogen and stored in liver or muscle cells
What is Amylose?
An unbranched chain of glucose linked by α(1→4) bonds; forms a helical shape.
What is Amylopectin?
A branched glucose polymer
Main chain: α(1→4) bondsBranches: α(1→6) bonds
What is Glycogen?
A highly branched glucose polymer, similar to amylopectin but more branched and larger.