Protein Structure and Denaturation Basics
1/23
Earn XP
Description and Tags
AA&P CH
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
24 Terms
Protein Denaturation
The process where proteins lose their higher-order structure due to environmental changes, resulting in a loss of function. Egg whites turn white when cooked.
Albumins
Proteins found in egg whites that have a specific 3D shape maintained by bonds between amino acids.
Hydrophobic Amino Acids
Amino acids that repel water and tend to clump together in a denatured protein to avoid water.
The four levels of protein structure.
Primary, Secondary, Tertiary, and Quaternary.
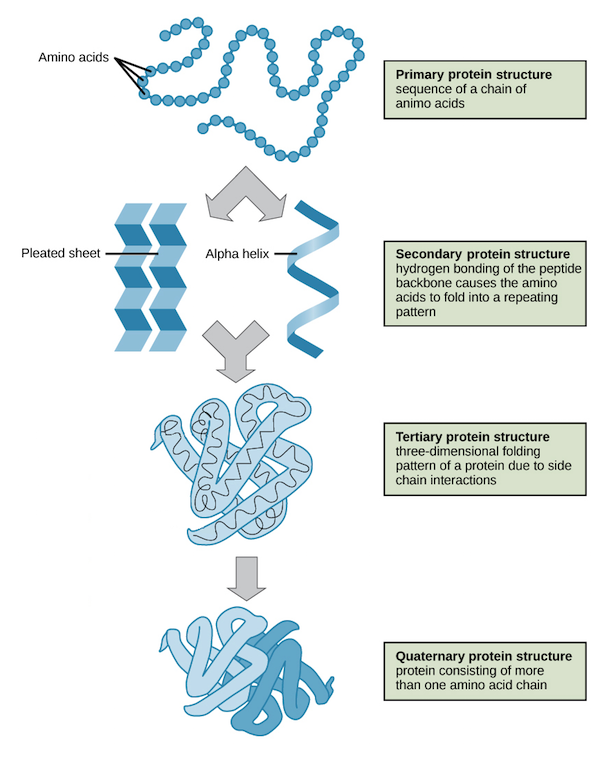
Primary Structure
The sequence of amino acids in a polypeptide chain, determining the protein's identity and function.
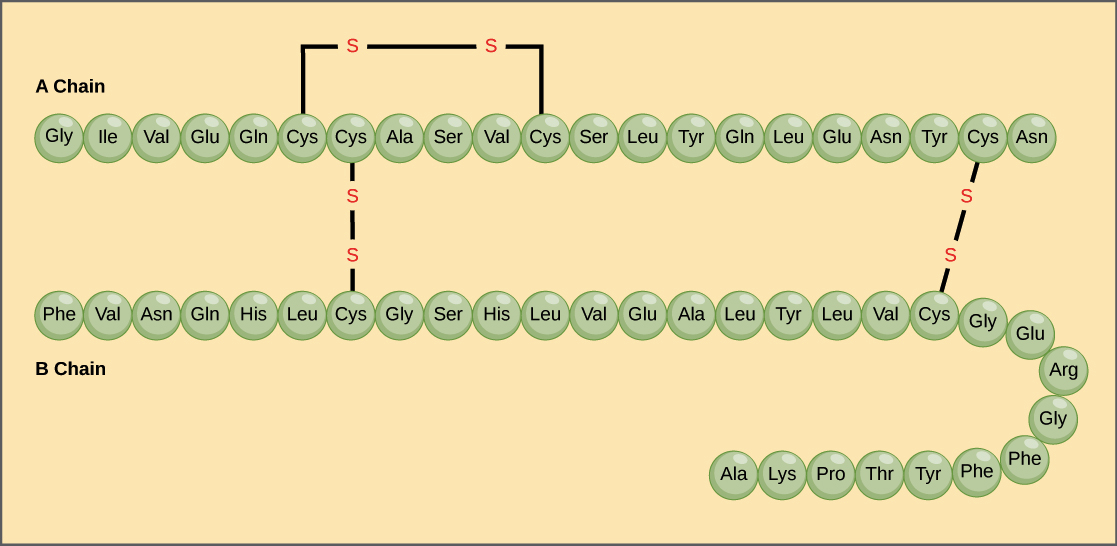
Primary Structure example:
The hormone insulin consists of two polypeptide chains, A and B, each with its own unique amino acid sequence. The A chain starts with glycine at the N-terminus and ends with asparagine at the C-terminus, differing from the B chain's sequence.
Sickle Cell Anemia
A genetic condition caused by a single amino acid change in hemoglobin (the protein that carries oxygen), leading to distorted red blood cells in the shape of a crescent moon.
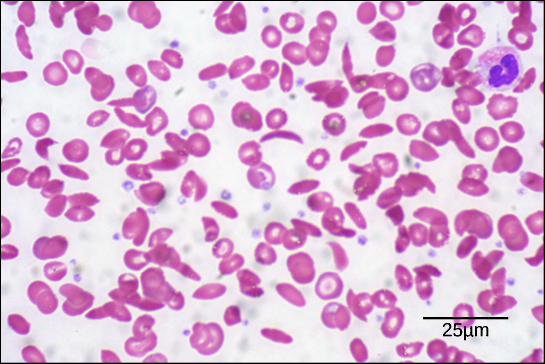
Sickle Cell Symptoms include:
These sickled cells become stuck in blood vessels, impairing blood flow and causing symptoms such as breathlessness, dizziness, headaches, and abdominal pain.
Sickle Cell example with Glutamic acid.
Image: In this condition, glutamic acid, the sixth amino acid in the hemoglobin β chain, is replaced by valine.
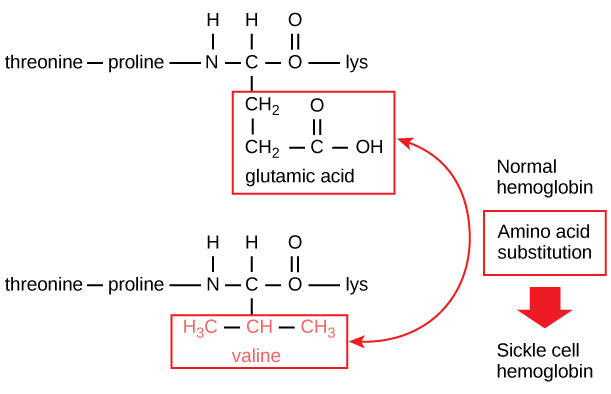
What is the difference between Normal vs. Sickle-Cell Hemoglobins?
Only two amino acids out of about 600.
Hemoglobin
Consists of two α chains and two β chains, each containing approximately 150 amino acids.
Secondary Structure
Local folded structures within a polypeptide chain, resulting from interactions between atoms of the backbone (polypeptide chain excluding the R group).
Common types of secondary structures
Include alpha helices and beta sheets. Both structures are stabilized by hydrogen bonds between the carbonyl O of one amino acid and the amino H of another.
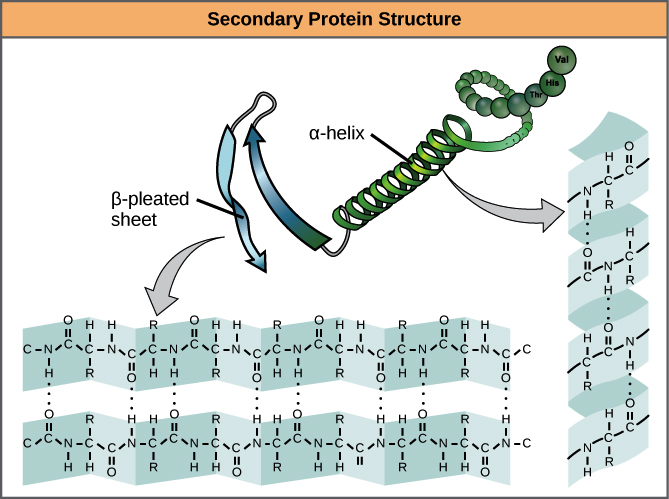
α Helix
The carbonyl (C=O) of one amino acid forms a hydrogen bond with the amino H (N-H) of an amino acid four positions down the chain.
How does the alpha helix perform?
The bonding pattern pulls the polypeptide chain into a spiral shape, with each turn containing 3.6 amino acids. The R groups extend outward from the α helix, allowing them to interact with other molecules.
β Pleated Sheet
A type of secondary structure formed by aligned segments of the polypeptide chain stabilized by hydrogen bonds.
How does the beta pleated sheet perform?
The hydrogen bonds form between the carbonyl and amino groups of the backbone, while the R groups extend above and below the plane of the sheet. Strands can be parallel or anti-parallel.
Why are some amino acids are more likely to be found in α-helices or β pleated sheets?
For example, proline is often called a "helix breaker" due to its unique R group, which creates a bend in the chain and disrupts helix formation. Amino acids with large ring structures in their R groups, such as tryptophan, tyrosine, and phenylalanine, are commonly found in β pleated sheets.
Tertiary Structure
The overall 3D shape of a polypeptide, determined by interactions between the R groups of amino acids.
What R-group interactions contribute to tertiary structure?
Hydrogen bonding
Ionic bonding
Dipole-Dipole bonding
London dispersion bonding
Include: Hydrophobic interactions and disulfide bonds, which are covalent linkages between the sulfur-containing side chains of cysteines, act as molecular "safety pins," holding parts of the polypeptide together
Quaternary Structure
The structure formed when multiple polypeptide chains or subunits come together to form a complete protein.
Quaternary Structure examples:
Hemoglobin: Carries oxygen in the blood and consists of two α and two β subunits.
DNA polymerase: Synthesizes new DNA strands and is composed of ten subunits.
Denaturation
The loss of a protein's structure due to environmental changes, which may be reversible or irreversible. Denatured proteins are usually non-functional.
Chaperone Proteins (Chaperonins)
Proteins that assist in the folding of other proteins, particularly after denaturation.