metal reactivity
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
Structure of metals
All metals have a giant metallic lattice structure with strong electrostatic forces of attraction between the metal ions (positive) with the sea of delocalised and mobile electrons (negative)
Physical properties of metals
Good conductors of heat and electricity
Usually have high melting and boiling points (Exocet Grp 1 and mercury)
Have high densities
Malleable (can be hammered into different shapes)
Ductile (can be drawn into wires without breaking)
Reasons for good conductors of electricity and heat
The sea of delocalised electrons act as mobile charge carriers and conducts heat
Have high melting and boiling points (except group 1 and mercury )
They have giant metallic lattice structures with strong electrostatic forces of attraction between metal ions and sea of delocalised and mobile electrons which require large amounts of energy to overcome
High densities
Metal ions are closely packed together in the solid state
Malleable And ductile
Atoms are orderly arranged in layers and these layers of atoms can slide over each other easily when a force is applied.
Alloy
A mixture of a metal with other elements (non metal and metal). They are stronger and more corrosion resistant
Examples of alloy
Mild steel; —> mixture of iron and carbon (used to make car bodies and machinery)
Stainless steel—> mixture of iron chromium nickel and copper (used to make cutlery , surgical instruments)
Why are alloys stronger and harder than pure metals
Alloys are made up of atoms of different sizes which disrupts the orderly arrangement of atoms making it hard for the layers of atoms to slide over one another when a force is applied. On the other hand, atoms are orderly arranged in layers . The layers of the atoms can slide easily over on another when a force is applied .
Reactivity Series table
Potassium
Sodium
Calcium
Magnesium
Aluminium
Carbon
Zinc
Iron
Tin
Lead
Hydrogen
Copper
Silver
Gold
The reactivity series can be used to predict
Chemical behaviour of a metal
Position of an unfamiliar metal in the reactivity series
The more reactive the metal is the more electrons they lose easily to form cations
Reactivity of metals can be compared by these reactions
Metal +cold water —> metal hydroxide and hydrogen
Metal + steam —> metal oxide and hydrogen
Dilute hydrochloric acid + metal —> meta chloride + hydrogen
Reaction with cold water (potassium)
Reacts very violently . Potassium darts around and sizzles on the surface of the water . Effervescence of colourless , odour less gas that extinguishes lighted splint with a ‘pop’ sound.
Reaction with cold water (sodium)
Reacts violently . Sodium darts around and sizzles on the surface of water .Effervescence of colourless , odour less gas that extinguishes lighted splint with a ‘pop’ sound
Reaction with cold water (calcium)
Reacts readily .Effervescence of colourless , odour less gas that extinguishes lighted splint with a ‘pop’ sound
Reaction with cold water (magnesium )
Reacts very slowly. Effervescence of colourless , odour less gas that extinguishes lighted splint with a ‘pop’ sound
Aluminium when reacting with water , stem or hcl
No visible reaction because aluminium reacts readily with oxygen in the air to form an insoluble protective layer of aluminium oxide which prevents it from further reaction
Reaction with water from carbon onwards
No reaction
Reaction with steam (potassium-calcium)
Reacts explosively
Reaction with steam (magnesium)
Reacts violently. Bright white glow produced, grey solid turns white
Reaction with steam (zinc)
Reacts readily. Hot grey zinc forms a yellow solid that turns white when cooled.
Reaction with steam (iron)
Grey iron turns red before forming a black solid
Reaction with steam (lead-gold)
No reaction
Reaction with dilute hydrochloric acid (Potassium , sodium , calcium, magnesium )
Potassium +sodium = reacts explosively
Calcium = violently
Magnesium = rapidly
All produce Effervescence of colourless , odour less gas that extinguishes lighted splint with a ‘pop’ sound
Reaction with hcl (zinc, iron)
Zinc= moderately fast
Iron = slowly
All produce Effervescence of colourless , odour less gas that extinguishes lighted splint with a ‘pop’ sound
Reaction with dilute hcl (lead-gold)
No reaction
Displacement reactions Definition
A more reactive metal can displace a less reactive metal from its salt solution
Eg . Iron + copper sulfate what is the displacement reaction
Iron sulfate + copper. Because iron is more reactive and loses electrons more readily. Hence, iron will displace copper from copper sulfate solution to form iron sulfate and copper
Colour of group 1 and 2 metals
Colourless
Colour of salts of copper and iron
Copper —> blue (green)
Iron —> Fe(ii)) green , Fe(iii) yellow/ brown
Action of heat on carbonates
The more reactive the metal, the more thermally stable is it carbonate . Hence, more difficult to decompose the carbonate by heat
When potassium and sodium carbonate are heated what happened
They do not decompose because they are very reactive metals and hence their carbonates are very thermally stable
Calcium , magnesium Zin , iron , lead , copper carbonate upon heating what happens
Decomposes into metal oxide and carbon dioxide on heating
Zin carbonate —> zinc oxide colors
Carbonate → white solid
Zinc oxide __> yellow when hot , white when cold
Copper carbonate —> copper oxide colour
Carbonate —> green
Oxide—> black
Silver carbonate Upon heat
Decomposes into silver and carbon dioxide on heating
Reaction between a metal and the oxide of another metal
A more reactive metal can reduce a less reactive metal from its oxide
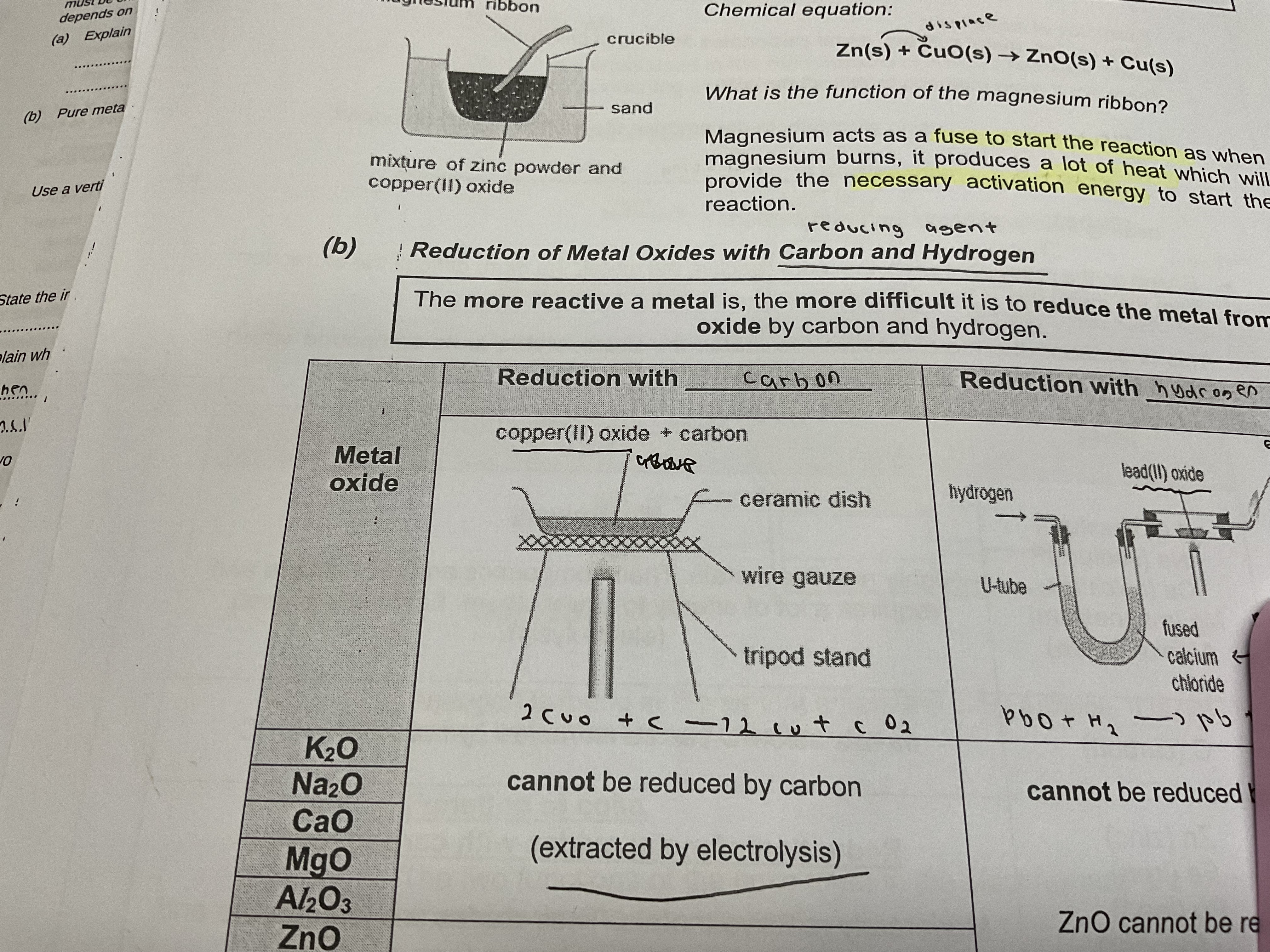
Magnesium ribbon function in reduction of metal oxides
Acts as a fuse to start the reaction as hen magnesium burns it produces a lot of heat which will provide the necessary activation energy to start the reaction
Reduction of metal oxides with carbon and hydrogen (reducing agents)
The more reactive a metal is , the more difficult it is to reduce the metal from its oxide by carbon and hydrogen
Reduction with carbon (Potassium to aluminium oxide)
Cannot be reduced
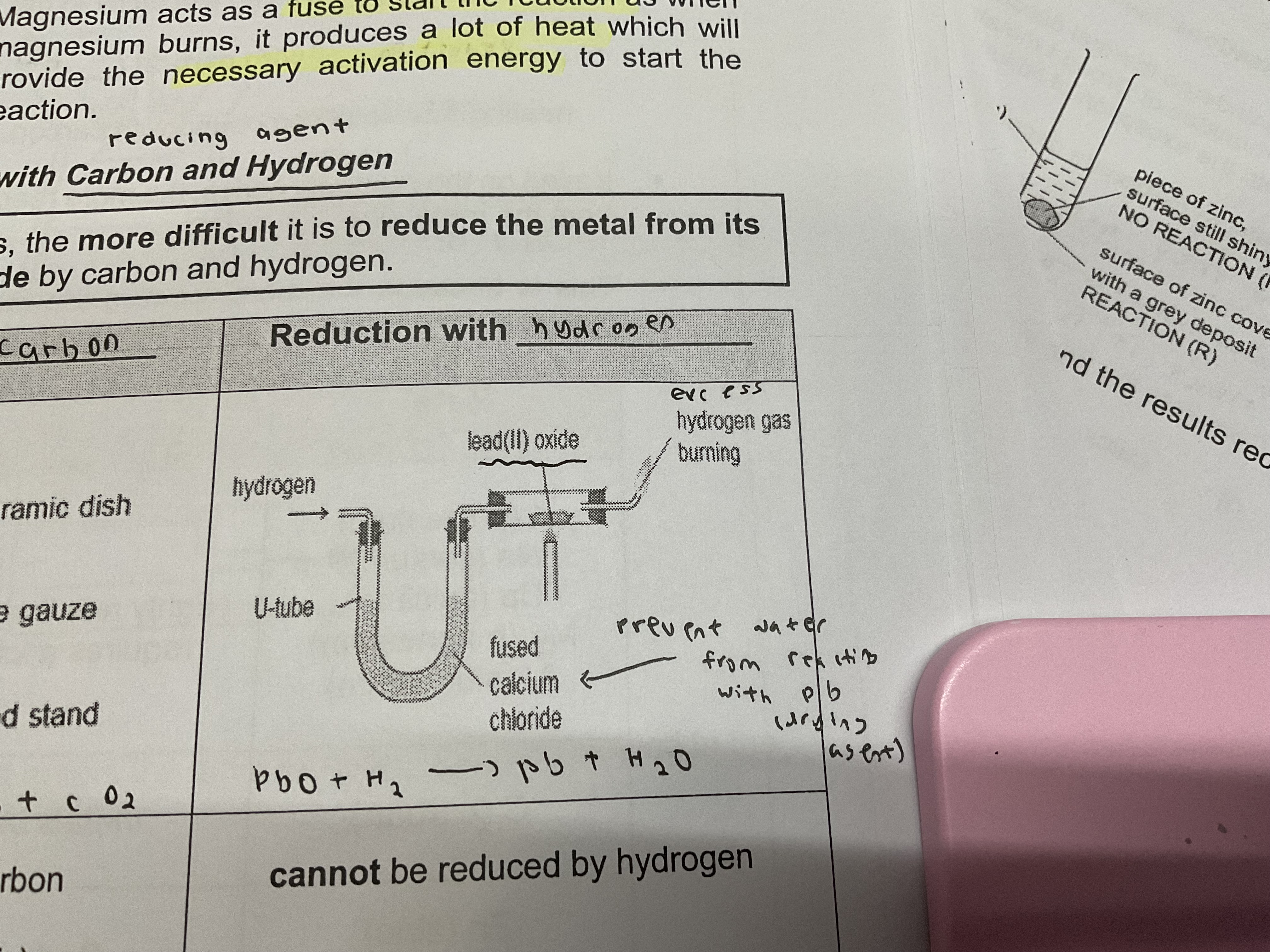
Reduction with carbon (zinc to copper oxide)
Reduction by carbon to form carbon dioxide and metal
Reduction with carbon and hydrogen on silver oxide
It can be decomposed by heating alone without any reducing agent
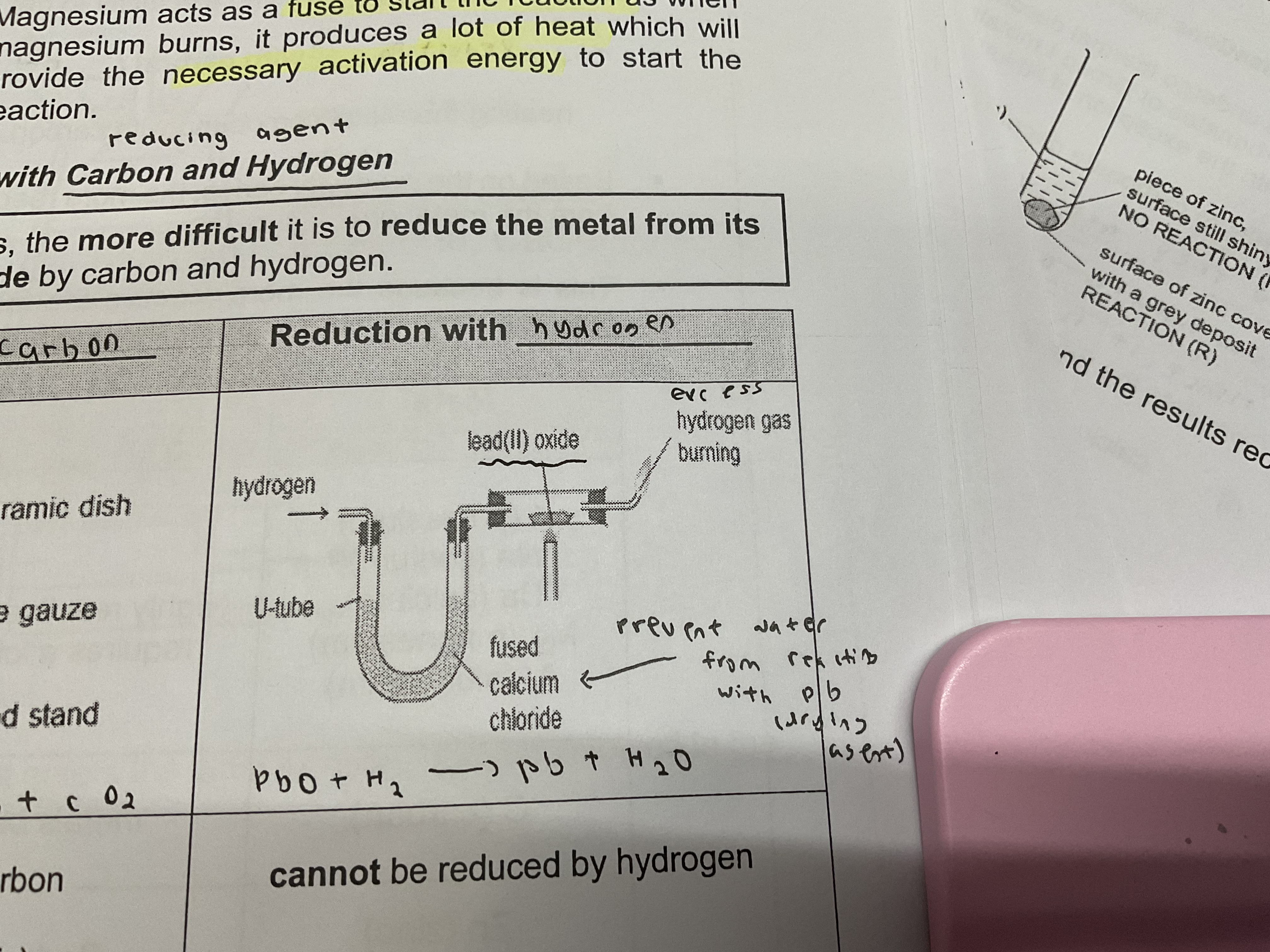
Reduction with hydrogen (Which cannot be reduced)
Potassium , sodium , calcium , magnesium , aluminium, zinc
Reduction with hydrogen (which can be reduced)
Reduced by hydrogen to form metal + steam