Unit 5: Electrochemistry
1/29
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Electrochemical reaction
Transfer of electrons from one substance to another.
2Al (s) + 3 Cu2+(aq) → 2Al3+(aq) + 3Cu (s)
OXIDATION: loss of electrons
Al → Al3+ + 3e-When a substance becomes oxidized, it becomes more positively charged because it is losing electrons (negatively charged).
REDUCTION: gain of electrons
Cu2+ + 2e- → CuWhen a substance becomes reduced, it becomes more negatively charged because it is gaining electrons.
REDOX reactions
Every reduction reaction must be accompanied by an oxidation reaction since electrons must be transferred somewhere.
2Al (s) + 3 Cu2+(aq) → 2Al3+(aq) + 3Cu (s)
OXIDIZING AGENT: reactant reduced (gains e-) during a reaction.
→ Cu2+ is the OXIDIZING AGENT because it causes Al to become oxidized.REDUCING AGENT: reactant oxidized (loses e-) during a reaction.
→ Al is the REDUCING AGENT because it causes Cu2+ to become reduced.
Oxidation number
The apparent charge that the atom would have if all of the bonds to the atom were completely ionic.
Atoms in elemental form = 0 (ex. N2, Na, O2)
Simple (monoatomic) ions = the charge on ion (ex. Fe2+ has an oxidation number of +2)
Li+, Na+, K+, and all other group 1 ions have oxidation number of +1.
Ca2+, Ba2+, Mg2+, and all of the group 2 ions have oxidation numbers of +2.
F-, Cl-, Br-, I- (halogens) are usually -1.
There are exceptions, especially in covalent compounds.
Hydrogen = +1 (except in metallic hydrides such as NaH or BaH2 where it is -1).
Oxygen = -2 (In peroxides, H2O2, it is -1)
Oxidation numbers of other atoms are assigned so that the sum of the oxidation numbers (positive and negative) equals the net charge on the molecule or ion.
Rule of OXIDATION/REDUCTION
OXIDATION = loss of electrons = increase in oxidation number.
If the number of attached oxygen atoms increases → Oxidation
REDUCTION = gain of electrons = decrease in oxidation number.
If the number of attached oxygen atoms decreases → Reduction
Predicting Spontaneous Reactions
In general, metals (exceptions Cu, Ag, Hg, and Au) are found in the bottom right half of the table (reducing agents).
In general, halogens and oxyanions (oxygen-containing anions) are found in the upper left half of the table (oxidizing agents).
Some metals, such as Fe, Sn, Cr, Hg, and Cu, have more than one common oxidation number and thus have more than one half-reaction.
Some ions (Cu+, Sn2+, Fe2+) appear on both sides of the table and can behave as oxidizing agents or reducing agents.
H2O2 can be an oxidizing agent or a reducing agent.
Half-reactions, including H+, must be carried out in acidic solutions.
→ Ex: MnO4- + 8H+ + 5e- → Mn2+ + 2H2O (acidic permanganate solution)
OXIDIZING AGENTS gain electrons, tend to be CATIONS (+) OR NONMETALS.
Stronger oxidizing agents are located on the upper left, have a greater tendency to gain electrons (reduce).
REDUCING AGENTS lose electrons, tend to be ANIONS (-) OR METALS.
Stronger reducing agents are located on the lower right, have a greater tendency to lose electrons (oxidize).
Spontaneous reactions occur when there is:
an oxidizing agent (reduction) and a reducing agent (oxidation), and
the oxidizing agent must be above the reducing agent.
Balancing Half Reactions
Balance all major atoms other than oxygen and hydrogen.
Balance OXYGENS by adding water (H2O) molecules.
Assume that solutions are acidic and balance HYDROGENS by adding H+.
Balance the CHARGE by adding electrons (e-).
(If BASIC solutions) Add equal numbers of hydroxide ions (OH-) to both sides of the equation and cancel out the H+ as water.
Balanced redox reactions DO NOT SHOW ELECTRONS and the NUMBER OF ATOMS AND THE TOTAL CHARGE are balanced on both sides of the equation.
DISPROPORTIONATION: same chemical undergoes oxidation and reduction.
Balancing Redox Equations Using Oxidation Numbers
Calculate the changes in oxidation numbers for the elements that undergo oxidation and reduction.
Balance increase and decrease in oxidation number change.
Balance oxygen with H2O and hydrogen with H+.
Redox Titrations - Oxidizing agents
One of the most common oxidizing agents used in redox titrations is Acidic Potassium Permanganate, KMnO4. You can acidify the reaction flask by adding concentrated acid such as sulphuric acid (H+ source).
MnO4- + 8H+ + 5e- → Mn2+ + 4H2O; E° = 1.49VThe K+ in KMnO4 is left out because it is a spectator ion.
In permanganate titrations, the MnO4- acts as its own indicator.
MnO4- is purple while Mn2+ is colourless. When MnO4- (purple) is added to a reducing agent, it is converted to Mn2+ (colourless). Once all the reducing agent has been oxidized by MnO4-, any additional MnO4- will remain purple.
Redox Titration - Reducing agents
Commonly used reducing agent is NaI or KI (I- is a RA). A large number of substances can oxidize I- to I2 according to the half-reaction:
Unknown OA + e- → Reduced form of OA
2I- → I2 + 2e-
Titrations involving I- are indirect and involve 2 consecutive steps:
I- is added in excess and is oxidized to I2 by the unknown oxidizing agent.
The I2 produced in the first step is titrated with a reducing agent of known concentration, such as thiosulphate ion S2O32- according to this reaction:
2S2O32- + I2 → S4O62- + 2I-
The concentration of the I- can be determined from the [I2], and this is used to determine the concentration of the unknown oxidizing agent.
I2 that is produced is a brown colour in the solution. Before any starch is added, enough RA (e.g., S2O32-) is added until the brown colour is faint.
Starch is used as an indicator for the I2 titration. The starch-I2 complex produces a dark blue solution. When I2 is titrated with S2O32-, the dark blue complex disappears (because it turns to I-). The endpoint of the titration is indicated by the complete disappearance of the blue colour.
Adding starch at the start of the titration step will interfere with the titration process because I2 binds fairly tightly to starch.
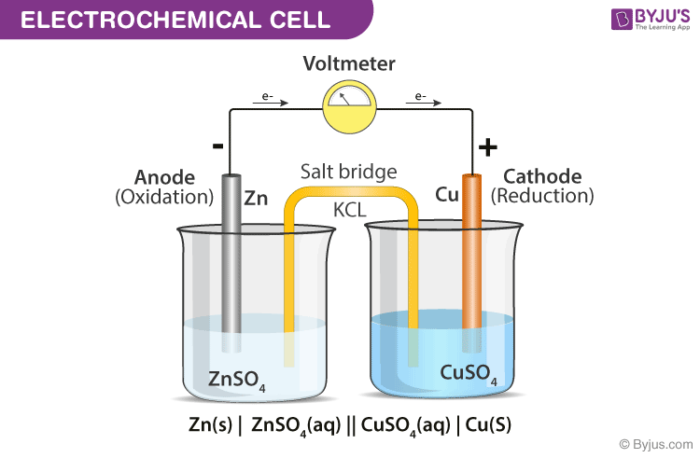
Electrochemical cells
An ELECTROCHEMICAL CELL (galvanic or voltaic cell) is a chemical system in which a SPONTANEOUS redox reaction can produce useful electrical work.
Cu2+ + Zn → Cu + Zn2+
Cu2+ is reduced to Cu, and Zn is oxidized to Zn2+.
A spontaneous reaction will occur when zinc metal is placed into a solution of CuSO4; however, no usable energy is produced because the energy is lost as heat.
Can be used to produce electricity if the two half-reactions are made to occur in separate containers or CELLS.
Electrodes
ANODE is the electrode where OXIDATION occurs.
CATHODE is the electrode where REDUCTION occurs.
AN OX CARED
ANode → OXidation
CAthode → REDuction
Cu2+ + Zn → Cu + Zn2+
Zinc metal loses electrons and becomes oxidized to zinc ions, Zn2+, at the ANODE, so it decreases in mass.
Copper ions, Cu2+, gain electrons and are reduced to copper metal at the CATHODE, so it increases in mass.
External Circuit
Electrons flow through the wire from the anode to the cathode.
“A comes before C in the alphabet”
Electrons produced at the zinc electrode (anode) flow through the wire to the copper electrode (cathode) where they reduce copper ions. The electrons will continue flow through the wire until the redox reaction reaches equilibrium.
Connecting Cells
A SALT BRIDGE composed of a concentrated solution of strong electrolyte, usually KNO3 or KCl, or a porous membrane connects the two cells to complete the circuit.
The salt bridge keeps the two compartments electrically neutral by allowing ions (ex. K+ and NO3 from KNO3) to migrate between the cells.
Positive Zn2+ and Cu2+ ions (cations) are attracted to the cathode.
Negative NO3- and SO42- ions (anions) are attracted to the anode.
Voltage (Electrical potential)
WORK DONE PER ELECTRON TRANSFERRED.
The tendency of electrons to flow in an electrochemical cell.
Since electrons cannot flow in an isolated half-cell, an individual half-cell voltage cannot be determined. However, the difference in electrical potentials between two half-cells can be measured.
A ZERO-POINT is ABITRARILY defined on the voltage scale. The voltage for the HYDROGEN HALF-CELL is defined to be:
2H+ + 2e- → H2 ; E° = 0.00V
STANDARD STATE
An electrochemical cell is said to be at the STANDARD STATE if
it is at 25°C, and
all gases are at 101.3 kPa (1atm), and
all elements are in their standard states (normal phases at 25°C), and
all solutions involved in the cell have a concentration of 1.0 M.
STANDARD REDUCTION POTENTIALS
Positive reduction potentials indicate that the half-reaction has a greater tendency to be reduced than the hydrogen half-cell.
Negative reduction potentials indicate that the half-reaction has a lower tendency to be reduced than the hydrogen half-cell. The oxidation reaction will occur when these half-reactions are connected to a hydrogen half-cell.
The value of an oxidation potential is the negative of the reduction potential (opposite signs).
The electrochemical cell potential is determined by adding the half-cell voltages for the reduction potential and oxidation potential.
If E° is POSITIVE for a redox reaction, the reaction is expected to be SPONTANEOUS.
If E° is NEGATIVE for a redox titration, the reaction is NON-SPONTANEOUS.
SURFACE AREA of the electrode
The SURFACE AREA of an electrode does not affect the voltage of the cell. This is because the CONCENTRATION OF THE SOLID IS CONSTANT.
Increasing the surface area of a solid electrode has no effect on the concentration of the solid.
No equilibrium shift and no change in half-cell potential.
Increasing the surface area of an electrode increases the AMERAGE (Rate of electron flow), but not the voltage.
Increasing the surface are of the electrode, also increases the length of time that the cell can operate.
Le Chatelier’s Principle
Cu2+ + 2e- ⇌ Cu(s) ; E° = +0.34 V
If [Cu2+] > 1.0 M, the equilibrium shifts right and the reduction potential increases.
→ E > +0.34 VIf [Cu2+] <1.0 M, the equilibrium shifts left and the reduction potential decreases.
→ E < +0.34 V
Notice that the “°” is omitted from the E° symbol since it is NOT AT STANDARD STATE.
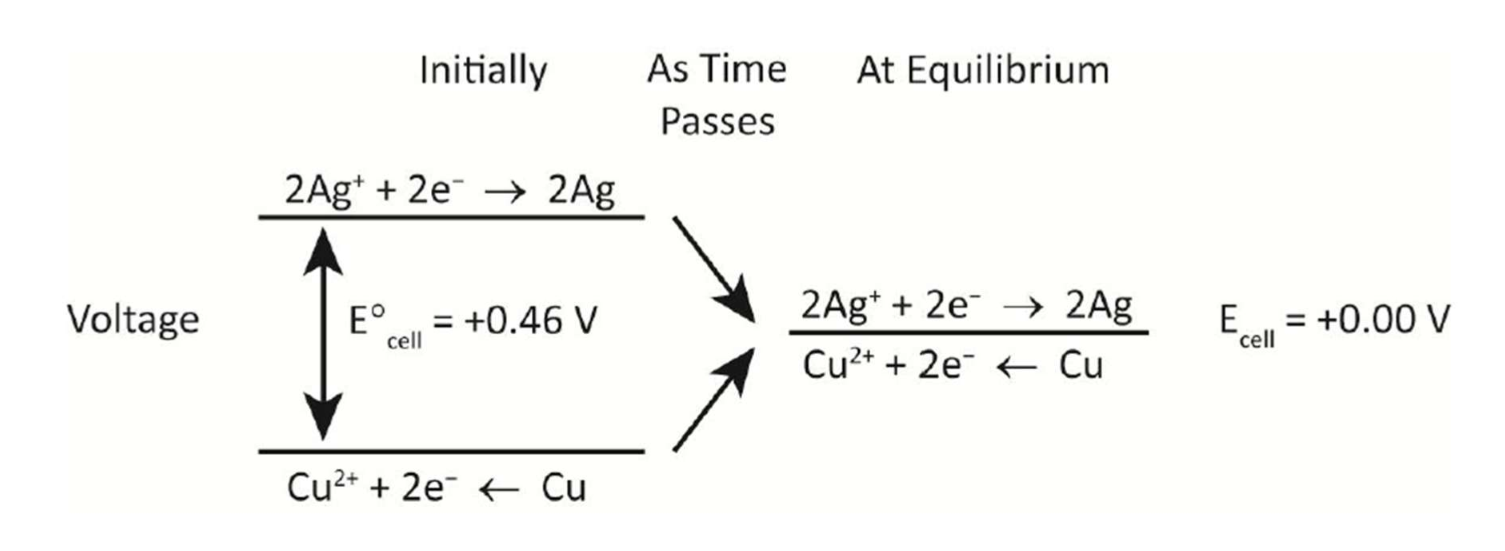
Electrochemical cells NOT AT EQUILIBRIUM
2Ag+ + Cu → 2Ag + Cu2+ ; E° = +0.46 V
Initially, this reaction has a great tendency to form products. As the cell operates, using up reactants and forming products, two effects are found:
REDUCTION REACTION:
2Ag+ + 2e- → 2Ag ; E < + 0.80 V
The [Ag+] decreases as the reaction occurs, and so the reduction potential decreases.
OXIDATION REACTION:
Cu2+ + 2e- ← Cu ; E° > + 0.34 V
The [Cu2+] increases as the reaction occurs, so the reduction potential Cu2+ + 2e- → Cu increases. The tendency for reduction increases as the cell operates.
When an electrochemical cell reaches EQUILIBRIUM, the VOLTAGE OF THE CELL IS 0.00 V.
SELECTING PREFFERED REACTIONS
STRONGEST OXIDIZING AGENT will become reduced.
STRONGEST REDUCING AGENT will become oxidized.
Note: Any ion capable of being reduced will be a SPECTATOR ION if there is another ion in the same solution which has a greater tendency to be reduced. Similarly, any ion capable of being oxidized will be a spectator ion if there is another ion in the same solution which has a greater tendency.
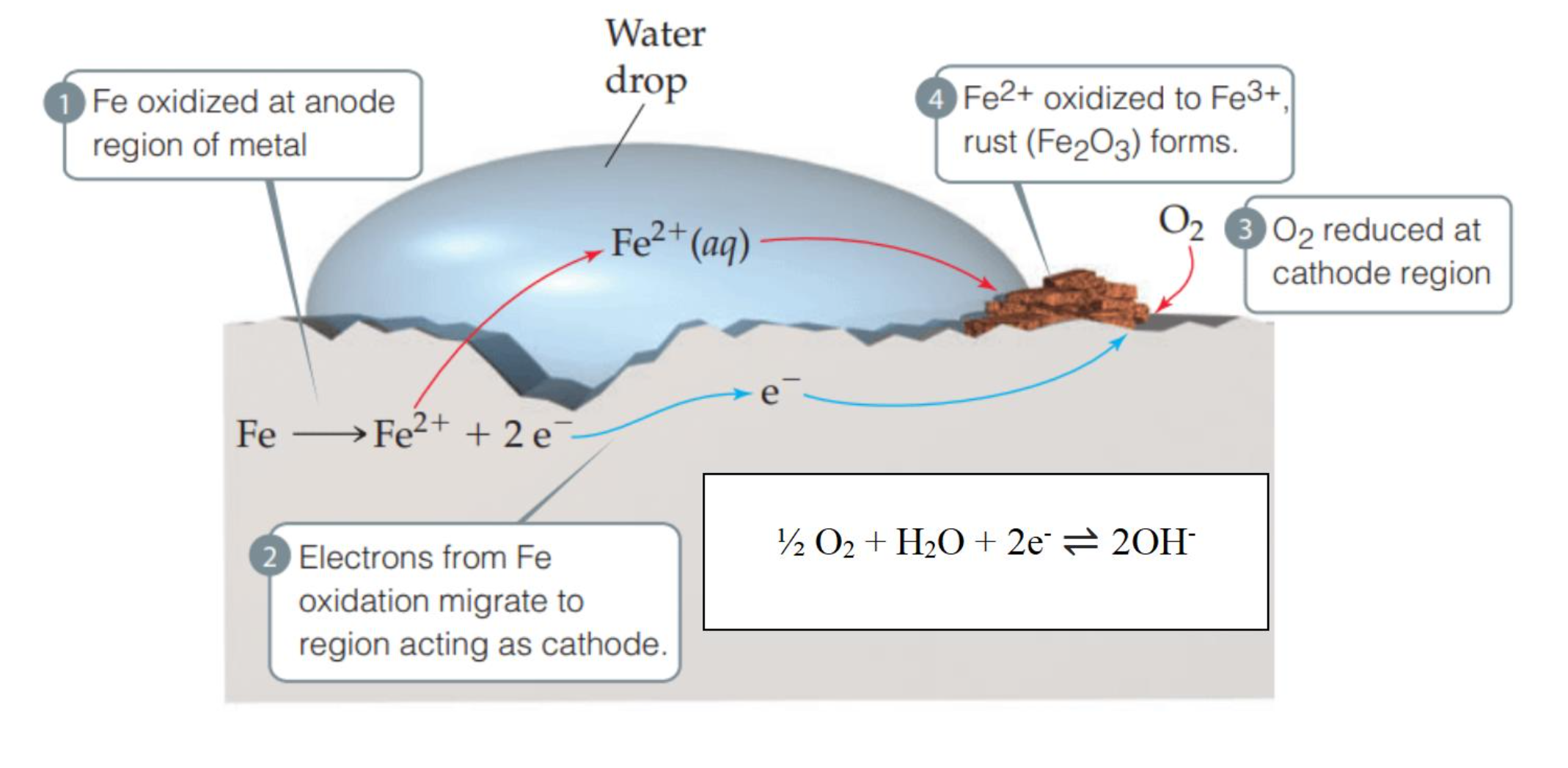
CORROSION OF METALS
Rusting is a common electrochemical reaction resulting from the oxidation of iron metal (Fe) by oxygen (O2) in the air (CORROSION = oxidation of a metal).
When iron is exposed to the atmosphere in the presence of water, the following spontaneous reactions occur.
Fe is oxidized at the anode (oxygen-poor) region of the metal.
Fe(s) → Fe2+ + 2e-Electrons from Fe oxidation migrate to the region, acting as a cathode.
O2 is reduced at the cathode (oxygen-rich) region at the outer surface.
½ O2 + H2O + 2e- ⇌ 2OH-Fe2+ oxidized to Fe3+, rust (Fe2O3) forms.
Fe(s) + ½ O2(g) + H2O(l) → Fe(OH)2(s)Fe(OH)2 is oxidized to a complex mixture of Fe2O3 and H2O by the O2 in the air. “Rust” is just Fe2O3.xH2O, where “x” is the variable.
“x” explains the different colours rust can have (red, brown, yellow, black) since different numbers of water molecules attached to the Fe2O3 will affect the colour of the component.
No salt bridge is needed because Fe2+ forms a precipitate with OH-.
Rate of corrosion
Rate of corrosion changes when iron is in contact with different metals.
When the metal is a stronger reducing agent than Fe (more readily oxidized), it is preferentially oxidized.
If the metal is a weaker reducing agent than Fe, such as Cu, it acts as a conductor of electrons and speeds up the rate of Fe oxidation.
Isolating the metal from the environment
Apply a protective layer to the metal, such as paint, plastic, or grease. If oxygen and water cannot reach the metal, it will not corrode.
Apply a metal that is corrosion-resistant to the surface of the original metal. Tin can be applied to the surface of steel cans. The tin is quickly oxidized to produce a thick layer of tin oxide, which protects the underlying metal from further corrosion.
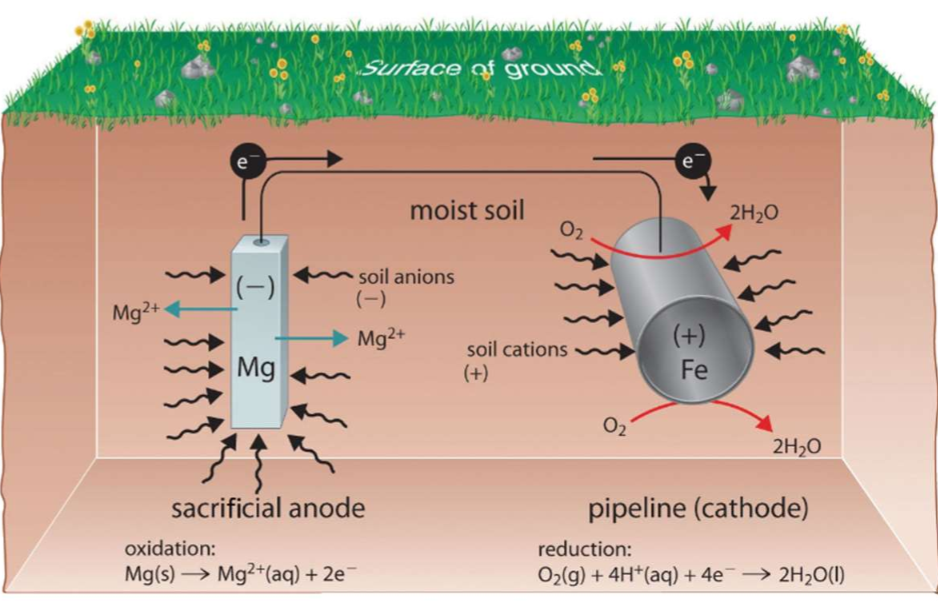
Electrochemical methods
Cathodic protection: protecting a substance from unwanted oxidation by connecting it to a stronger reducing agent.
Sacrificial electrodes using magnesium, for example, are used to protect underground tanks or pipes. Replacing the sacrificial electrodes is more cost-effective than replacing the iron objects they are protecting.
Change the condition of the surroundings: we can prevent corrosion by altering the reduction reaction that causes Fe to become oxidized
½ O2 + H2O + 2e- ⇌ 2OH-If oxygen is removed from the solution, the reduction potential decreases.
Increase [OH-] ions also causes the reduction potential to decrease (ie, alkaline environment).
Electrolysis
The process of supplying electrical energy to a molten ionic compound or a solution containing ions to produce a chemical change.
ELECTROLYTIC CELLS involve NONSPONTANEOUS redox reactions that require energy to occur (ENDOTHERMIC).
ELECTROCHEMICAL CELLS involve SPONTANEOUS redox reactions that release energy (EXOTHERMIC).
Electrolysis of Molten Binary Salts
Binary salts are made up of only two different elements (e.g., NaCl, KBr, MgI2, AlF3, etc.)
Molten binary salts, such as NaCl, contain only two kinds of ions.
Molten NaCl is NaCl(l), only Na+ and Cl- ions present.
NaCl(aq) is a solution that contains Na+, Cl-, and H2O.
No need for a salt bridge to keep the reactants separated since no spontaneous reaction will occur, although a barrier can be used to prevent products from mixing.
Electrolysis of Aqueous Solutions
Electrolysis of aqueous solutions must consider the possibility that H2O may be oxidized and/or reduced.
Electrolysis of aqueous solutions containing Cl- or Br- will produce Cl2 or Br2 at the anode.
Electrolysis of aqueous solutions containing Fe2+, Cr3+, or Zn2+ will produce Fe, Cr, or Zn at the cathode.
Determining the overall reaction
The STRONGEST OXIDIZING AGENT becomes reduced and the STRONGEST REDUCING AGENT become oxidized.
The preferred overall reaction will be the one requiring the LEAST voltage input.
OVERPOTENTIAL EFFECT
Higher potential than calculated must be supplied to cause electrolysis.
Causes include the nature of the electrodes, temperature, current, and time.
Difference between actual potentials required for electrolysis and the calculated potential is termed HALF-CELL OVERPOTENTIAL.
Overpotentials vary for each half-cell but the difference in potentials is very large for oxidation and reduction of neutral water.
ELECTROPLATING
An electrolytic process in which a metal is reduced or “plated out” onto an object connected to the cathode (e.g., coat something with metal).
The CATHODE is made out of the material that will receive the metal plating.
The ELECTROPLATING SOLUTION contains ions of the metal that is to be “plated” onto the cathode.
The ANODE may be made of the same metal that is to be “plated out” onto the cathode (This is normal, but an inert electrode can also be used).