Cofactors
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
Enzyme Cofactor
A non-protein chemical compound that assists enzyme activity, either by stabilizing structure or participating in the reaction.
cations are often cofactors
Inorganic Cofactor
A type of cofactor that consists of metal ions like Fe²⁺, Mg²⁺, Mn²⁺, Zn²⁺, or Cu²⁺.
Coenzyme
A organic or metalorganic type of cofactor that temporarily carries specific functional groups during enzyme-catalyzed reactions.
What is the difference between cofactors and coenzymes?
Cofactors include both inorganic ions and coenzymes, while coenzymes are only the organic or metalloorganic type.
many of the coenzymes are derivitives of adenosine
Phosphorylation
The addition of a phosphate group (often from ATP) to a molecule like a sugar, protein, or lipid, increasing its reactivity.
ATP does this
Kinase
An enzyme that transfers a phosphate group from ATP to a substrate, commonly used in signaling and metabolism.
ATP as a Cofactor
ATP helps facilitate reactions by donating phosphate groups, making target molecules more reactive (often by making them more electron-deficient).
Coenzyme A (CoA or CoASH)
A cofactor that functions as a carrier of acyl (acid) groups in metabolic reactions.
its derived from vitamin B5 (its the precursor)
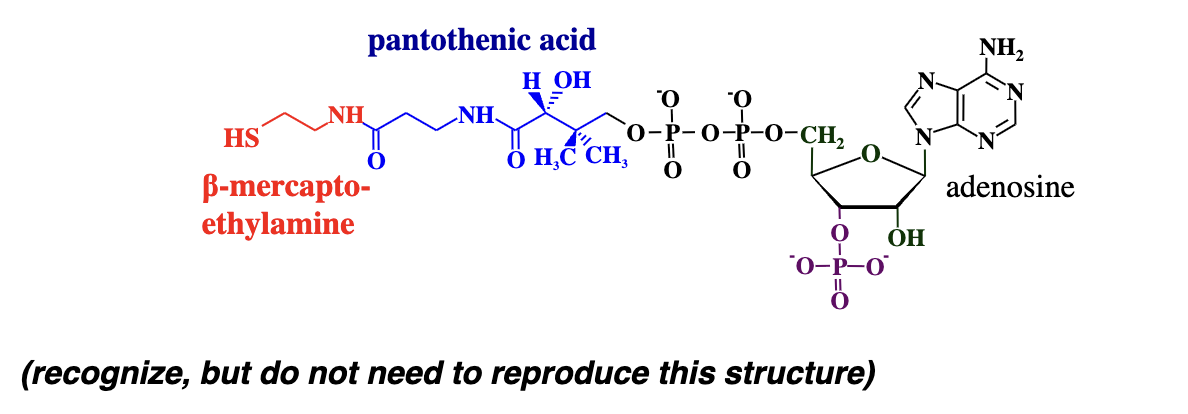
Coenzyme A (CoA or CoASH) has what kind of group that it attacks an acid with?
A thiol group (-SH) whihc is very reactive
the SH groups attacks the carbonyl carbon of carboxylic acid forming a thioester bond
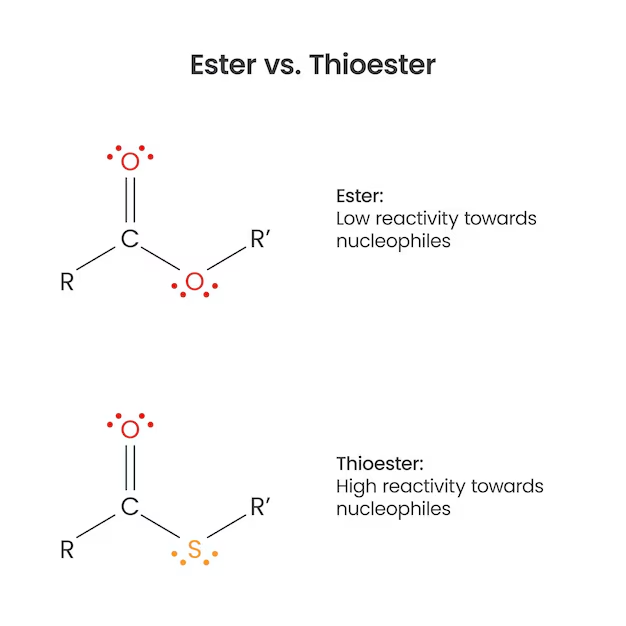
Thioester
A thioester is like a regular ester but oxygen is replaced with sulfer
they are high energy compounds, meaning thge bond is easily broken to drive other reactiosn forward.

the green part is the thioester bond
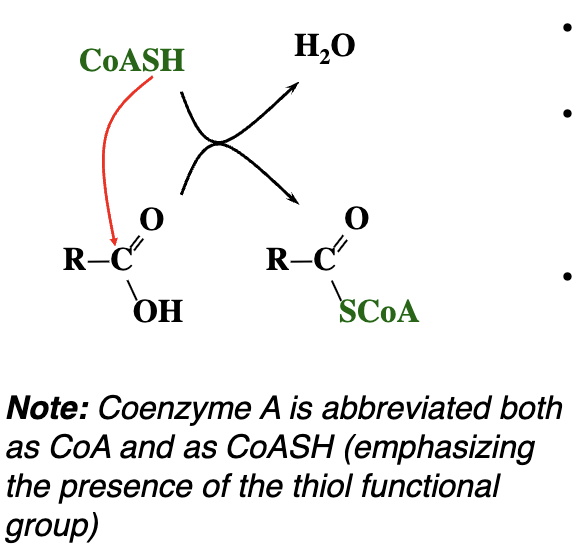
What does Coenzyme A (CoA or CoASH) do?
CoA forms thioester bonds with organic acids (e.g., acetyl group), producing high-energy compounds like acetyl-CoA. These activated molecules are used to transfer acyl groups in metabolic pathways.
Coenzyme A uses its thiol group to form a thioester bond with an acid, creating an acyl-CoA. This activates the acid, making it more reactive for further metabolic reactions.
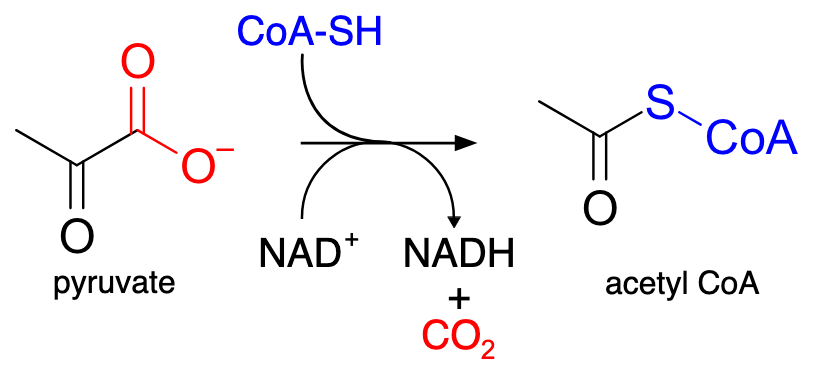
What is the difference between acyl-CoA and acetyl-CoA?
Acyl-CoA has a generic R group, while acetyl-CoA specifically has a CH₃ (methyl) group.
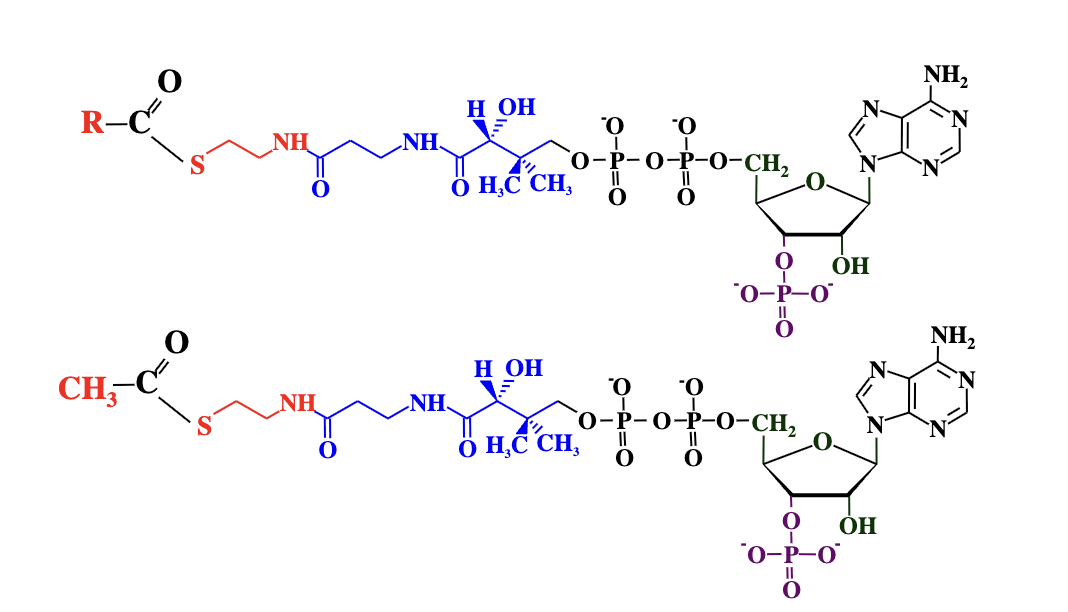
you can see after the S the coenzyme A
Electron carriers
A molecule like NAD⁺, NADP⁺, FAD, or FMN that accepts electrons during oxidation and transfers them in metabolic pathways.
the electrons that are removes from the substrates are transfered on to these cofactors reducing them and conserving the enery of oxidation.
NAD⁺ (Nicotinamide Adenine Dinucleotide) and NADP⁺ (Nicotinamide Adenine Dinucleotide Phosphate)
NAD⁺ and NADP⁺ are pyridine nucleotides derived from niacin (vitamin B3).
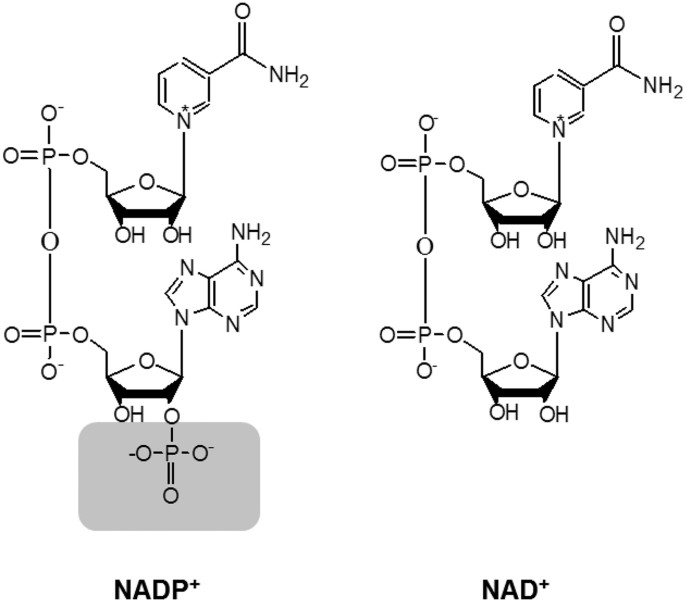
difference: NADP⁺ has an extra phosphate group on the 2′ position of the ribose attached to the adenosine. NAD⁺ has just an –OH at that position.
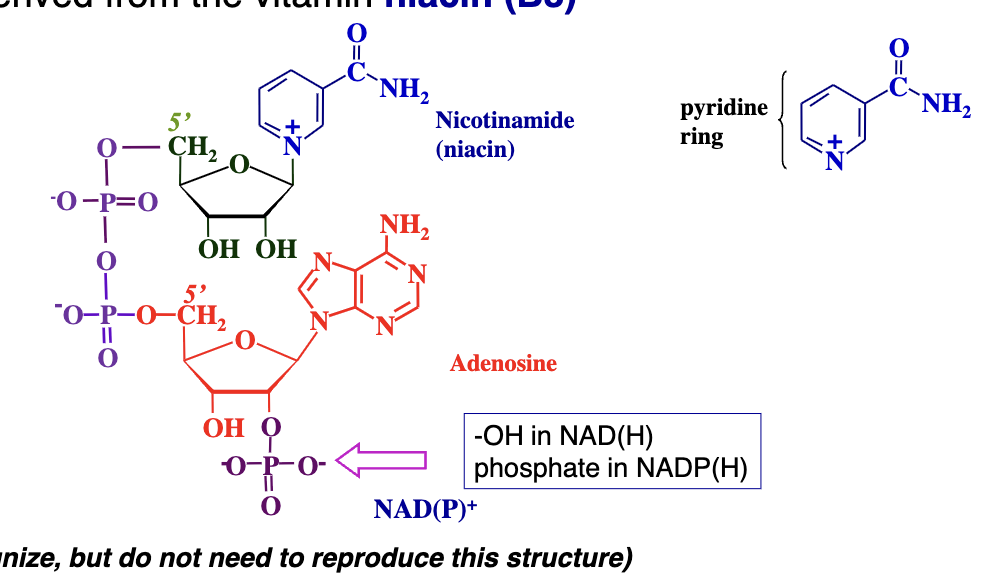
pyridine ring - 6 membered nitrogen containing ring in these carriers that can accept electrons
Where do redox reactions occur in NAD+ and NADP+?
the redox reactions happen on the nicotinamide ring, which includes the pyridine ring and its attached amide.
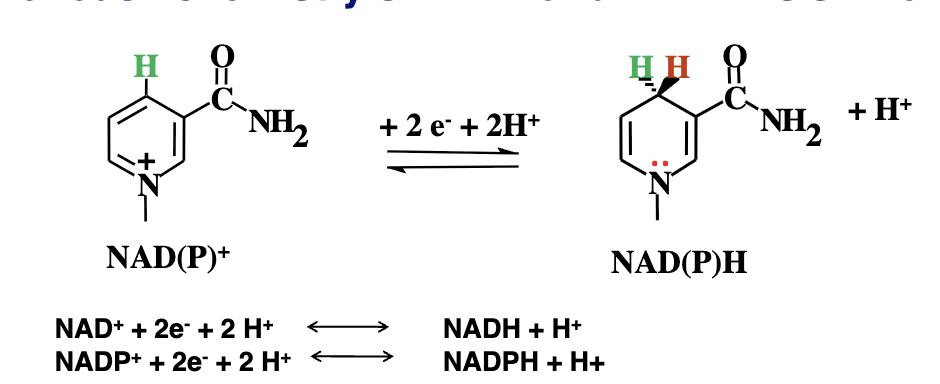
during oxidation of substrates (to give lectrons to these carriers) we see a dehydorgenation of 2 hydorgen atoms (1 proton H+ and one hydorgen ion H-)
What happens when NAD+ or NADP+ recieve the hydorgens from the substrate?
one hydrigen is removed as a proton into the acquoes environment and the other is accepted at the nicotinamide ring, becoming NADH or NADPH(the reduced form)
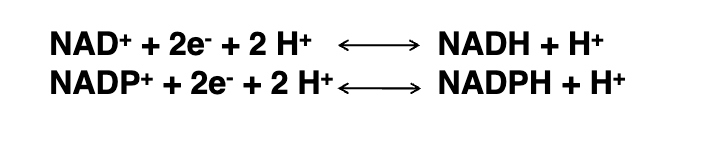
NAD⁺ vs. NADP⁺ charge
NADP⁺ has an extra phosphate group attached to the ribose ring that NAD⁺ lacks, adding extra negative charge.
Enzyme Specificity for NAD and NADP
Some enzymes specifically use NAD⁺, others specifically use NADP⁺, due to structural and charge differences.
NAD⁺ Cellular Role
Acts mainly as an oxidizing agent in catabolic pathways (e.g., fatty acid oxidation, TCA cycle).
NADPH Cellular Role
Acts as a reducing agent in anabolic (biosynthetic) pathways (e.g., fatty acid synthesis, steroid synthesis).
FAD and FMN → The flavin nucleotides
Flavin nucleotides derived from vitamin B2 (riboflavin); act as prosthetic groups tightly bound to enzymes. Electron carriers.
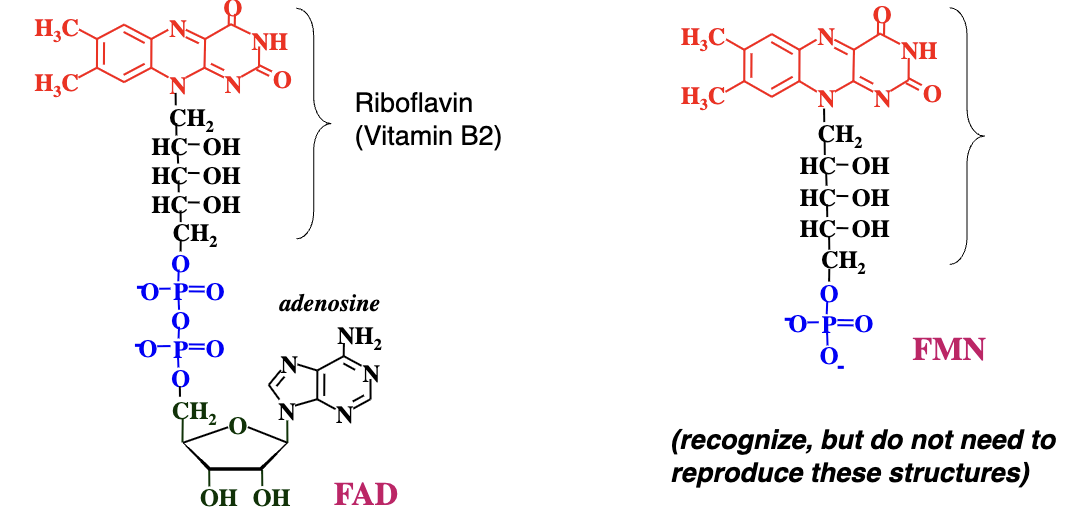
Prosthetic Group
A non-protein cofactor permanently bound to an enzyme essential for its activity.
Flavin Nucleotides Redox Flexibility
FAD and FMN can accept either one electron (as one hydrogen atom) or two electrons (as two hydrogen atoms) during oxidation reactions from substrates.
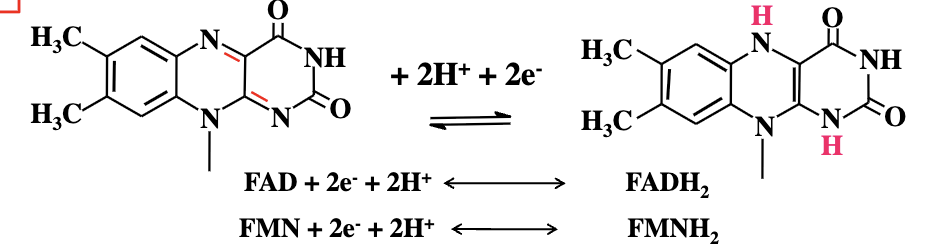
Fully Reduced Forms of Flavin Nucleotides
The fully reduced forms are FADH₂ and FMNH₂ after accepting two electrons and two protons.
Semiquinone Radical Forms
When flavins accept only one electron, they form stable semiquinone radical species: FADH· and FMNH·
notice how theres a dot after
this tells us that Flavin nucleotides exist in three states (oxidized, fully reduced and semiquinone radical)
Which is the stronger oxidizing agent? FAD or NAD+?
FAD, it has a higher standard reduction potential, and is tightly bound to enzymes as a prosthetic group allowing it to participate in reactions that NAD+ cannot.
B-vitamin cofactors: Which vitamin corresponds to which cofactor and what does it carry?
