Section L: Lecture notes: Biological Membranes
1/52
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
53 Terms
Lipids: Objectives
Objectives:
Biological roles of lipids
Structure and properties of storage lipids
Structure and properties of membrane lipids
Structure and properties of signaling lipids
4 General Types of Membrane Lipids: Phospholipids
Have hydroponic regions composed of two fatty acids joined to glycerol or sphingosine
4 General Types of Membrane Lipids: Glycolipids
Contain a simple sugar or a complex oligosaccharide at the polar ends
4 General Types of Membrane Lipids: Archaeal Tetraether Lipids
Have two very long alkyl chains ether-linked to glycerol at both ends
Archaebacteria: Bacteria that live in very extreme harsh environments like really high temperatures, really high salt conditions, or pH extremes.
Their biological membranes need to be extra strong, more than humans, b/c they live in these extreme environments.
4 General Types of Membrane Lipids: Sterols
Compounds characterized by a rigid system of four fused hydrocarbon rings
Ex: Cholesterol
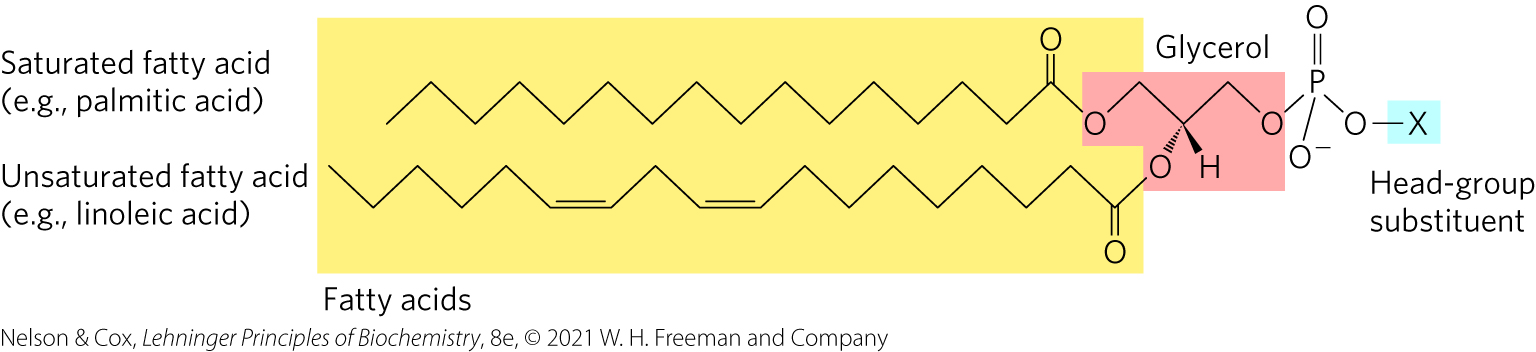
Glycerophospholipids (phosphoglycerides)
Membrane lipids in which two fatty acids are attached in ester linkage to the first and second carbons of glycerol, and a highly polar or charged group is attached through a phosphodiester linkage to the third carbon
Most important and abundant structural component of biological membranes
Similar to TAG (triacylglycerols)
But 1 key difference: Glycerophospholipids has the 3rd hydroxyl groups of that glycerol backbone is NOT attached to another fatty acid (see highlighted blue “x” in picture). Instead, an “x” is attached to the phosphate. (x = additional types of chemicals). This is called a “Head-group”.
Amphipathic molecules => self-assemble & come together in these belayers that form biological membranes.
Platelet-Activating Factor
an ether lipid that serves as a potent molecular signal
releases from leukocytes called basophils
stimulates platelet aggregation and serotonin release
plays a role in inflammation and the allergic response
Signaling molecule
Sphingolipids
large class of membrane phospholipids and glycolipids
have a polar head group and two nonpolar tails
Sphingosine itself is like a built-in fatty acid
contain no glycerol
contain one molecule of the long-chain amino alcohol sphingosine or one of its derivatives
Fatty acids that get attached are typically saturated FA
Linkage: amide
Not all sphingolipids = phospholipids
Most of them are not
Glycoplipids
Head group is a sugar
3 Type of Glycolipids: Cerbrosides
Have a single sugar linked to ceramide
those with galactose are found in the plasma membranes of cells in neural tissue
those with glucose are found in the plasma membranes of cells in nonneural tissues
3 Type of Glycolipids: Globosides
glycosphingolipids with 2+ (more than 1) sugars, usually D-glucose, D-galactose, or N-acetyl-D-galactosamine
Cerebrosides & Globosides are…
Neutral Glycolipids
they have no charge at pH 7
*Note: Cerebrosides & Globosides have simple sugars
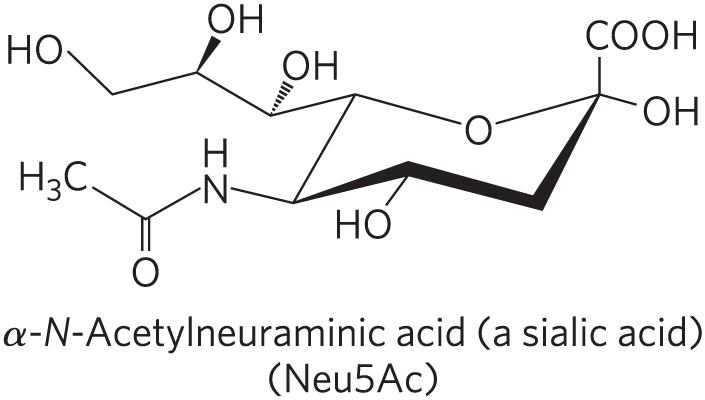
4 Type of Glycolipids: Gangliosides
JUST KNOW:
Not neutral
Complex branch sugars, where we have this negatively charged head group.
Extra info:
Have oligosaccharides as their polar head groups and 1+ residues of N-acetylneuraminic acid (Neu5Ac), a sialic acid, at the termini
1 sialic acid residue = GM (M for mono-) series
2 sialic acid residues = GD (D for di-) series
3 sialic acid residues = GT (T for tri-) series (and so on)
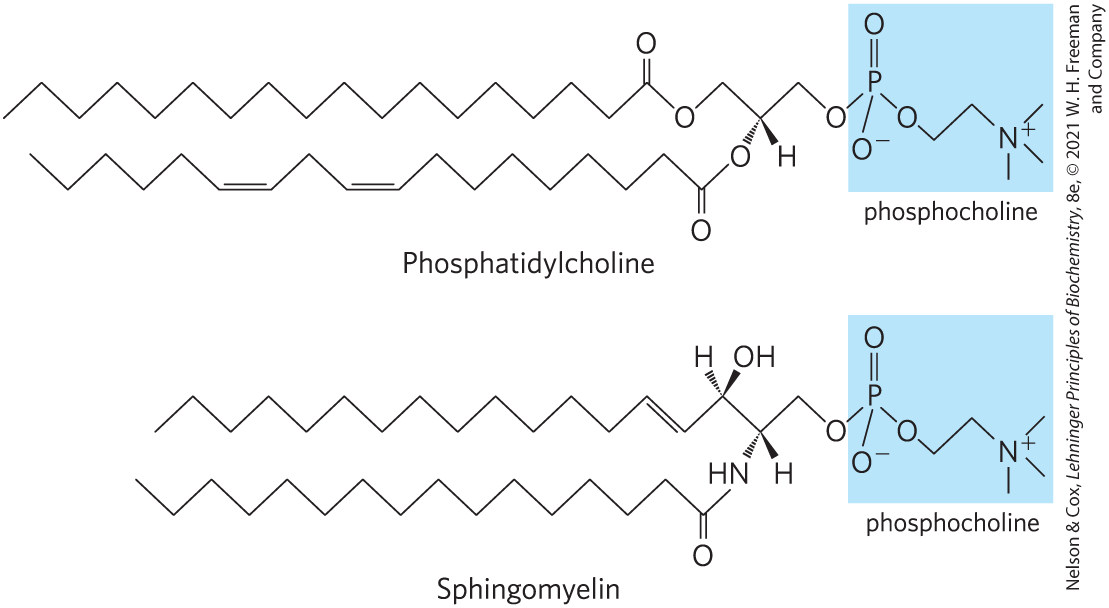
Sphingomyelins
Subclass of sphingolipids that contains phosphocholine or phosphoethanolamine as their polar head group
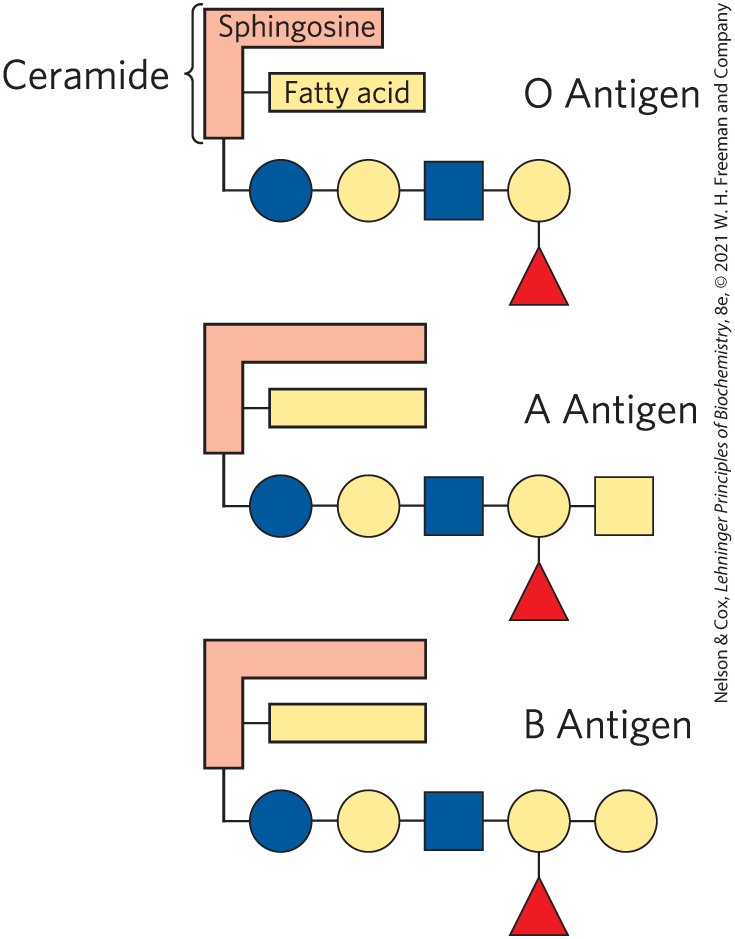
Sphingolipids @ cell surfaces are sites of biological recognition
Glycolipids, with these sugar head-groups, are typically on the outer leaflet of the membrane where sugars would face outside the cell.
These sugars are recognition sites for different types of molecules to bind
Some sphingolipids are glycolipids — but not all.
(Similar to how some sphingolipids are phospholipids — but not all.)
Expression of glycosyltransferase (enzyme) = attaches sugars to head-group
No expression = no sugar attachment => O-antigen
Just know: The head-group substituents differ because of this enzyme
Phospholipids & Sphingolipids are degraded in Lysosomes
phospholipases of the A type remove one of the two fatty acids
lysophospholipasesremove the remaining fatty acid
lysosomal enzymes catalyze the stepwise removal of sugar units of gangliosides
Many types of enzymes that break both Phospholipids & Sphingolipids
Abnormal Accumulations of Membrane Lipids-lysosomal storage diseases
Genetic defects in any of these hydrolytic enzymes leads to the accumulation of gangliosides in the cell
Need glycospingolipids, BUT they need it at the right amount, right place, at the right time.
Too much leads to buildup and causes diseases
Sterols Have Four Fused Carbon Rings
sterols = structural lipids present in the membranes of most eukaryotic cells
steroid nucleus: consists of four fused rings; almost planar; relatively rigid
cholesterol = major sterol in animal tissues
amphipathic
polar head group
nonpolar hydrocarbon body
membrane constituents
similar to stigmasterol in plants and ergosterol in fungi
Physiological Role of Sterols
Cholesterol as structural component of membranes
modulate fluidity and permeability
thicken the plasma membrane
no sterols in most bacteria
Mammals obtain cholesterol from food or synthesizeit de novo in the liver.
Cholesterol, bound to proteins, is transported to tissues via blood vessels.
Cholesterol in low-density lipoproteins (LDL) tends to deposit and clog arteries.
Many hormones are derivatives of sterols.
Male and female sex hormones
Sterols Serve as Precursors for Products with Specific Biological Activities
bile acids = polar derivatives of cholesterol that emulsify dietary fats in the intestine to make them more readily accessible to digestive lipases
Steroid Hormones Carry Messages between Tissues
Signaling lipids
Steroids = oxidized derivates of sterols
lack the alkyl chain attached to ring D of cholesterol
more polar than cholesterol
Steroid hormones move through the bloodstream (on protein carriers) to target tissues
Binding to highly specific nuclear hormone receptor proteins in the nucleus triggers changes in gene expression
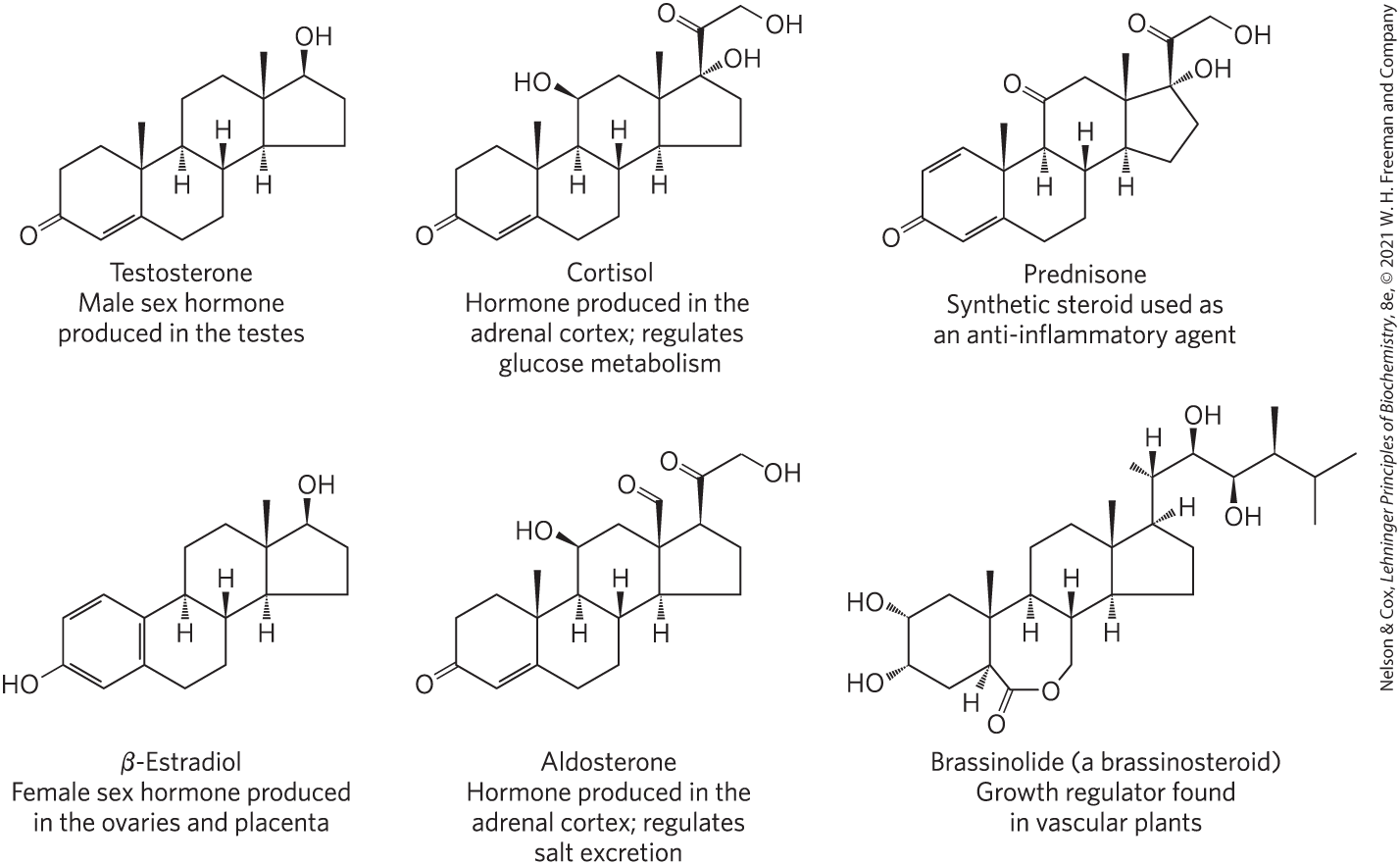
Steroids Derived From Cholesterol
Testosterone, Cortisol, Prednisone (synthetic steroids), β-Estradiol, Aldosterone, Brassinolide (plants)
Biologically Active Lipids
Are present in much smaller amounts than storage or structural lipids
Play vital roles as signaling molecules between nearby cells
Lipid-soluble vitamins (A, D, E, and K)
Phosphatidylinositol 4,5-Bisphosphate (PIP2)
in the cytoplasmic, inner leaflet of plasma membranes
serves as a reservoir of messenger molecules that are released in response to extracellular signals
phospholipase C hydrolyzes PIP2 to the second messengers IP3 and diacylglycerol (DAG), which in turn control downstream signaling effector enzymes or channels
Inositol sugar head-group
Glycerophospholipid
Signaling lipid
Eicosanoids Carry Messages to Nearby Cells
Paracrine hormones, substances that act only on cells near the point of hormone synthesis instead of being transported in the blood
Paracrine: act locally on cells near where they’re made
Signaling lipid
PUFA (polyunsaturated fatty acids)
Involved in:
reproductive function
inflammation, fever, and pain associated with injury or disease
formation of blood clots
regulation of blood pressure
gastric acid secretion
Eicosanoids Are Derived From Arachidonic Acid
4 major classes of eicosanoids:
prostaglandins
thromboxanes
leukotrienes
lipoxins
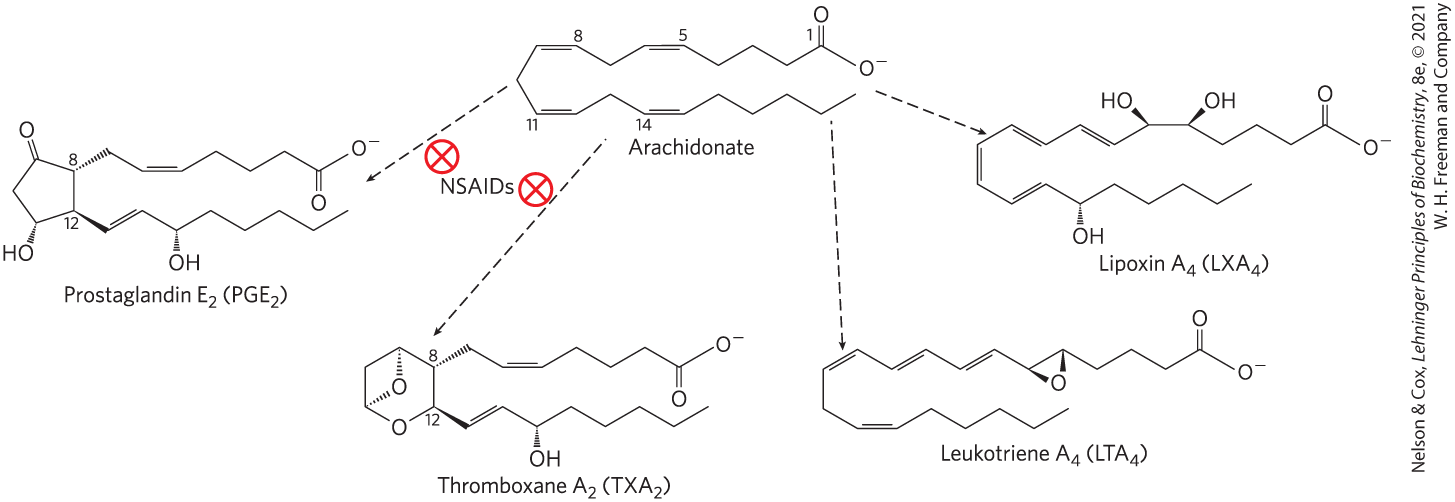
Prostaglandins (PG)
Class of eicosanoids that contain a five-carbon ring
array of functions:
stimulate contraction of the smooth muscle of the uterus
affect blood flow to specific organs, the wake-sleep cycle, and the responsiveness of certain tissues to hormones
elevate body temperature and cause inflammation and pain
Thromboxanes (TX)
Class of eicosanoids that have a six-membered ring containing an ether
produced by platelets (also called thrombocytes)
act in the formation of blood clots and reduction of blood flow to the site of a clot
Vitamins A and D Are Hormone Precursors
vitamins = compounds that are essential to the health of humans and other vertebrates but cannot be synthesized
So we get vitamins from diet
fat-soluble vitamins include the groups A, D, E, and K
“Fat kid KADE”
Vitamin D3 Production and Metabolism
calcitrol = hormone that regulates calcium uptake in the intestine and calcium levels in the kidney and bone
All-Trans-Retinoic Acid
signaling lipid
vitamin A1 (all-trans-retinol) = acts in processes of development, cell growth and differentiation, and vision
vitamin A1 or β-carotene can be converted enzymatically to all-trans-retinoic acid
all-trans-retinoic acid = retinoid hormone that acts through a family of nuclear receptor proteins to regulate gene expression
Vitamins E and K and the Lipid Quinones Are Oxidation-Reduction Cofactors
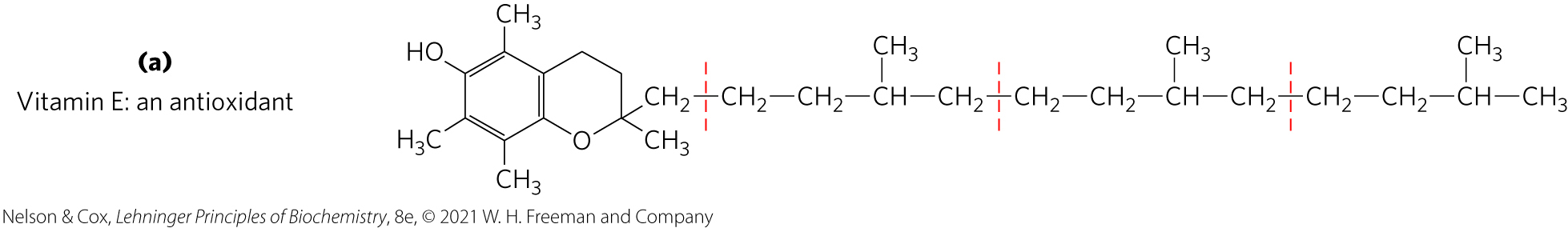
vitamin E = collective name for a group of lipids called tocopherols
tocopherols = hydrophobic compounds that contain a substituted aromatic ring and a long isoprenoid side chain
associate with cell membranes, lipid deposits, and lipoproteins
biological antioxidants
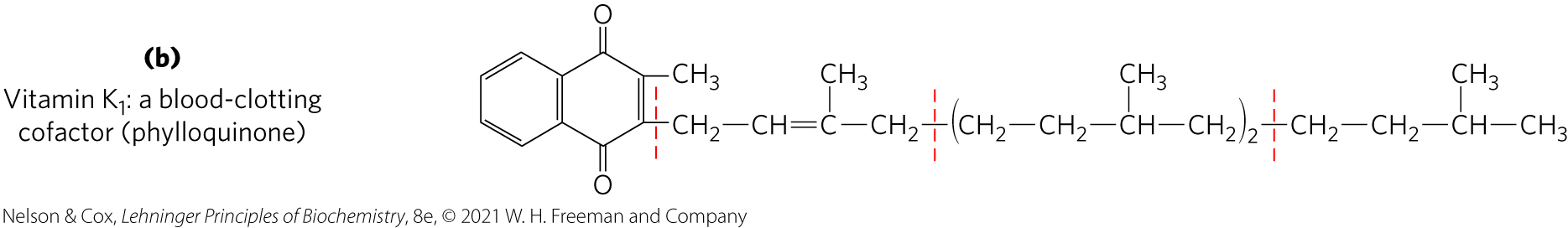
Vitamin K
vitamin K = contains an aromatic ring that undergoes a cycle of oxidation and reduction during the formation of active prothrombin, a blood plasma protein essential in blood clotting
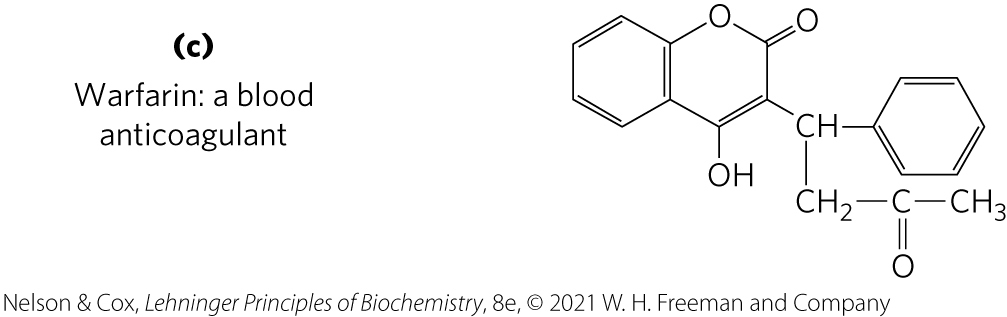
Ubiquinone and Plastoquinone
Ubiquinone (coenzyme Q) and plastoquinone = isoprenoids that function as lipophilic electron carriers in the oxidation-reduction reactions that drive ATP synthesis in mitochondria and chloroplasts, respectively
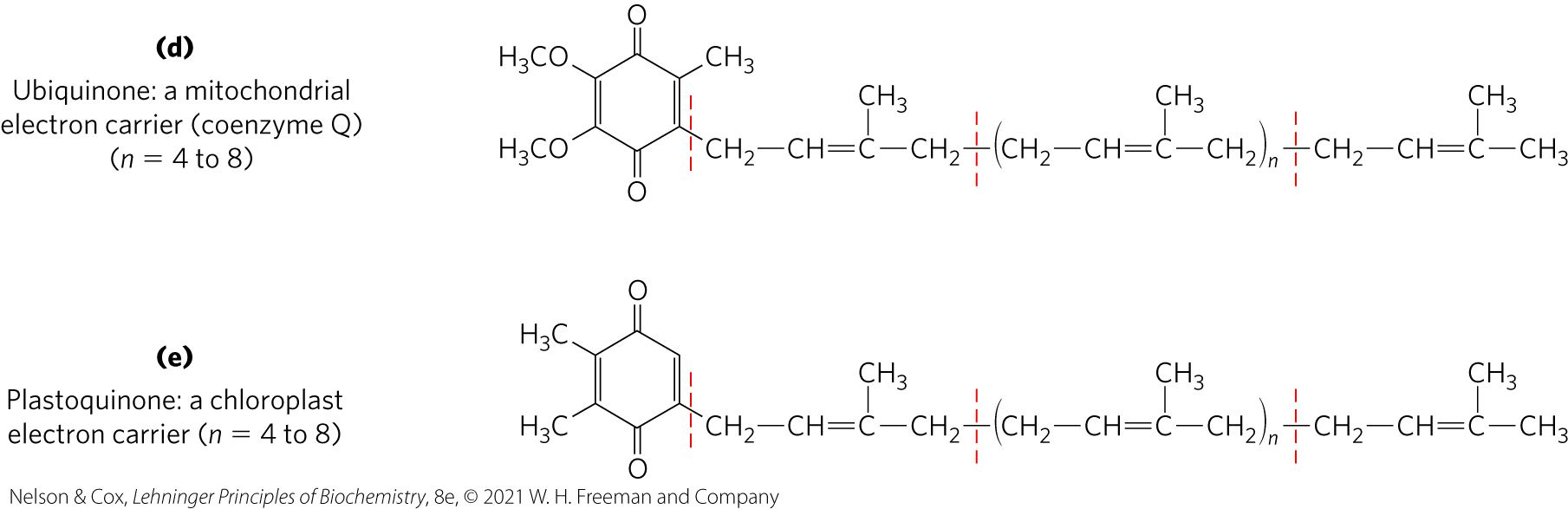
Dolichols Activate Sugar Precursors for Biosynthesis
dolichols = isoprenoid alcohols that activate and anchor sugars to cellular membranes
sugar groups are then used in the synthesis of complex carbohydrates, glycolipids, and glycoproteins
allow attached sugars to participate in sugar-transfer reactions
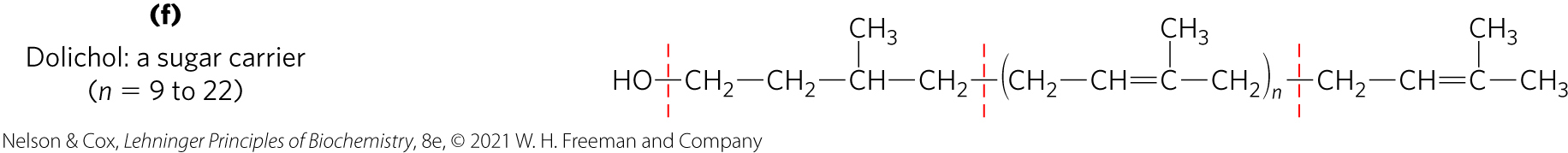
Working with lipids: A Commonly Used lipid Extractant is a Mixture of Chloroform, Methanol, and Water
mixture separates into two phases: methanol/water (top phase) and chloroform (bottom phase)
lipids remain in the chloroform layer
more polar molecules (proteins and sugars) partition into the methanol/water layer
Adsorption Chromatography Separates Lipids of Different Polarity
lipids in mixtures can be separated based on their polarity and interactions with polar materials such as silica, using adsorption chromatography methods such as HPLC or TLC
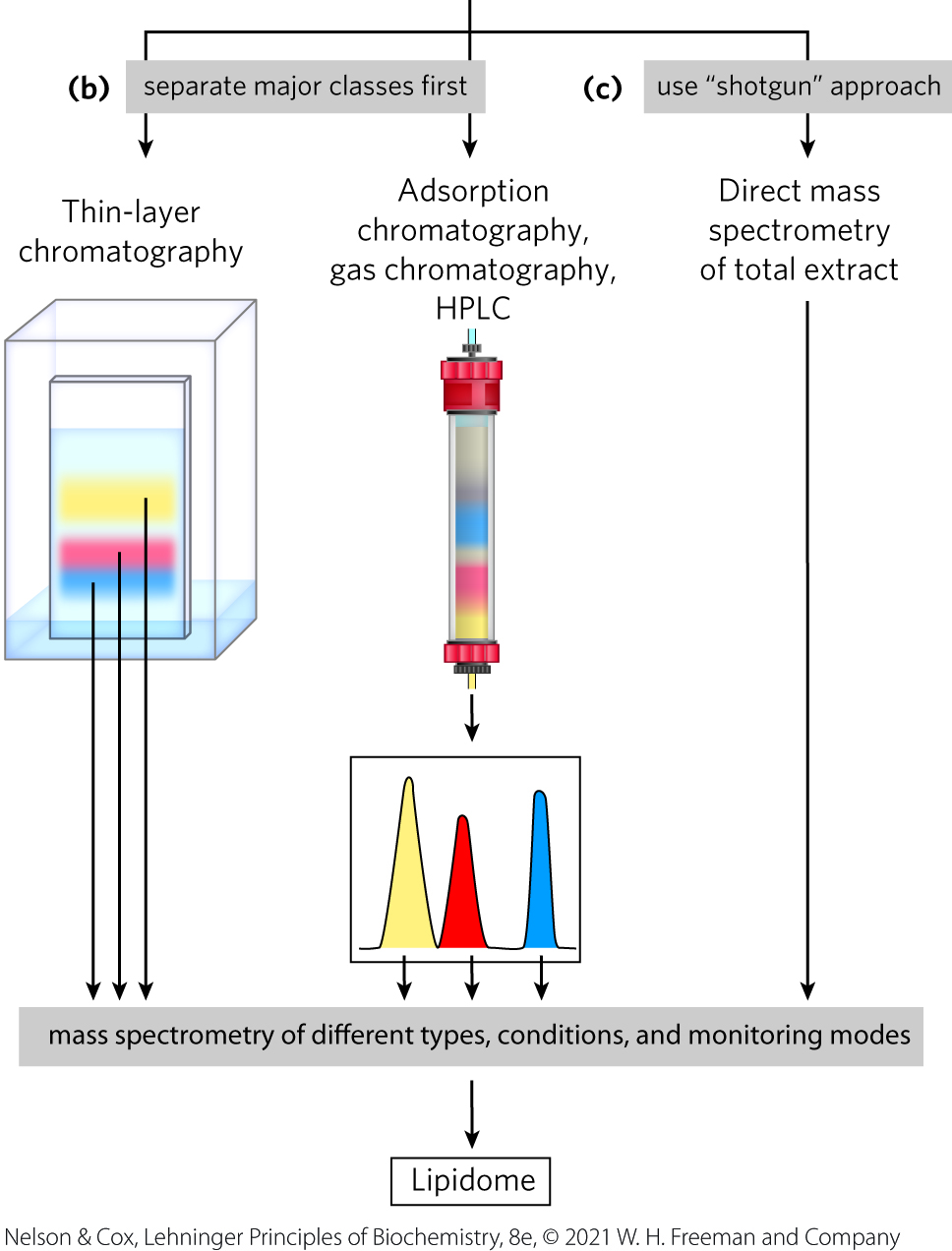
Lipids Summary
lipids are a structurally and functionally diverse class of molecules that are poorly soluble in water
triacylglycerols are the main storage lipids
phospholipids are the main constituents of membranes
Sphingolipids (& Glycosphingolipids) play roles in structure and in cell recognition
cholesterol is both a membrane lipid and the precursor for steroid hormones
some lipids carry signals from cell to cell and from tissue to tissue
Membranes and Transport: Objectives
Objectives:
The function of biological membranes
The structure and composition membranes
Physical properties and dynamics of membranes
Structure and function of membrane proteins
Transport across biological membranes
The Lipid Bilayer Is Stable in Water
Membrane = Lipid bilayers
Glycerophospholipids, Dphingolipids, and Sterols:
virtually insoluble in water
spontaneously form microscopic lipid aggregates when mixed with water
Hydrophobic interactions = the clustering of hydrophobic molecule surfaces in an aqueous environment to find the lowest-energy environment by reducing the hydrophobic surface area exposed to water
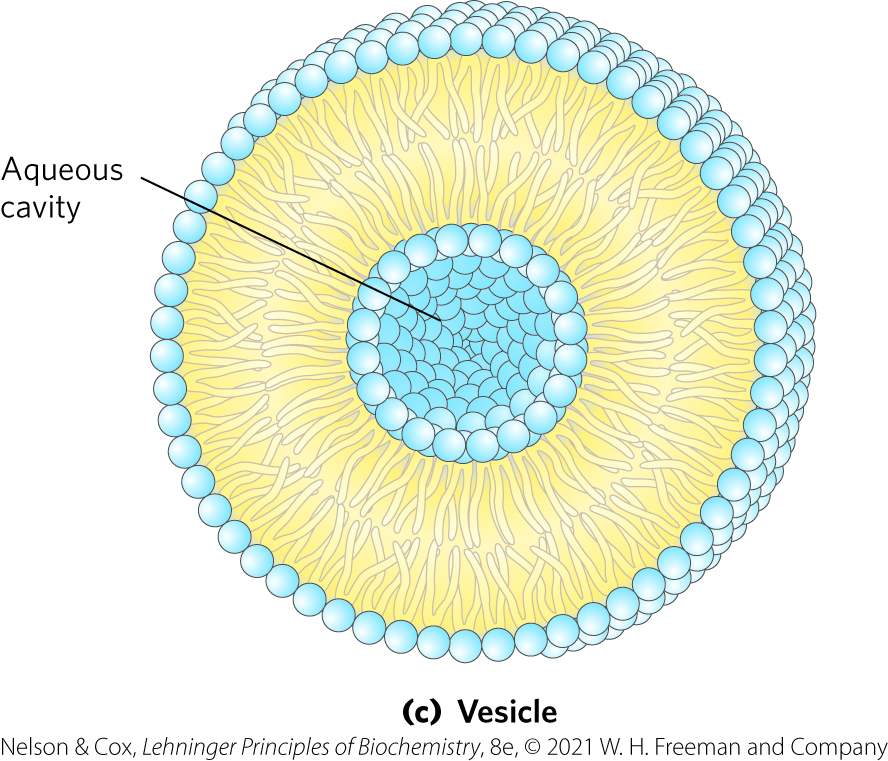
Vesicle Formation
Vesicle (liposome) = forms spontaneously when a bilayer sheet folds back on itself to form a hollow sphere
In simpler words: lipids bilayer sphere w/ aqueous cavity
Functions of Biological Membranes
Compartmentalization
permit shape changes that accompany cell growth and movement
permit exocytosis, endocytosis, and cell division
serve as molecular gatekeepers (selective barrier)
Bilayer Architecture Underlies the Structure and Function of Biological Membranes
Fluid mosaic = pattern formed by individual lipid and protein units in a membrane
pattern can change while maintaining the permeability membrane
most membrane proteins can move freely within the lipid bilayer, hence “fluid” in “fluid mosaic”
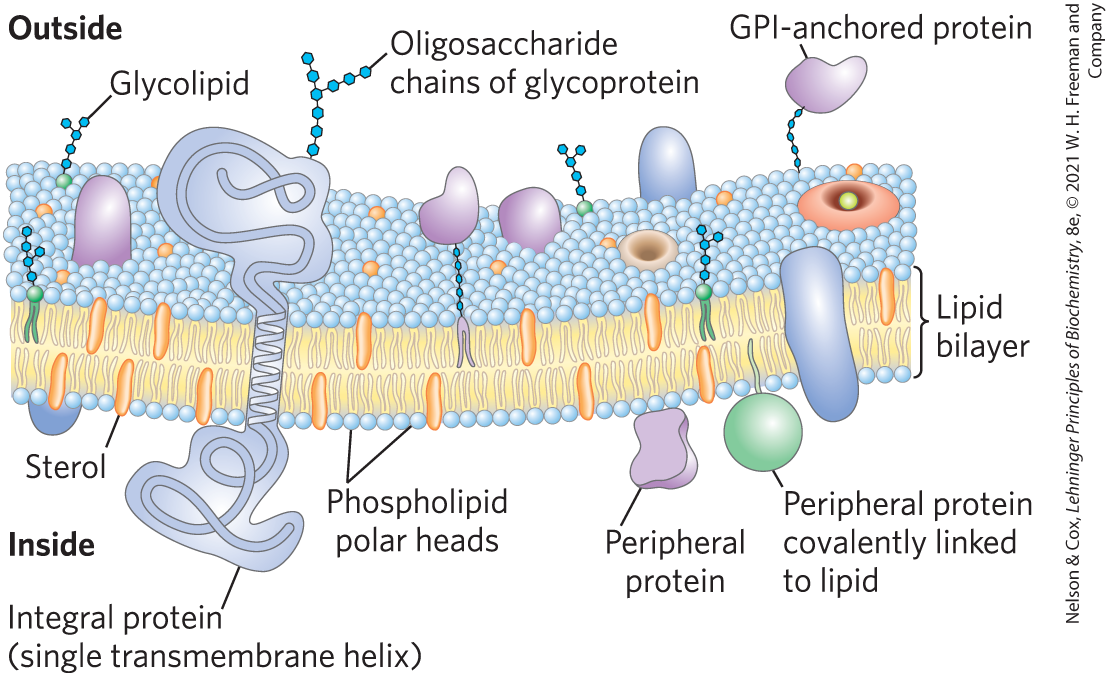
The Composition of Membranes
Lipid composition of membranes varies by:
organisms
tissues
organelles
Ratio of lipid to protein varies
type of phospholipid varies
abundance and type of sterols varies
lack of sterols in prokaryotes
cholesterol predominant in the plasma membrane, virtually absent in mitochondria
galactolipids abundant in plant chloroplasts but almost absent in animals
Where are Sphingolipids tend to be more enriched?
Outer leaflet in plasma membrane
sugar groups face outside of cell
Where are Glycerophospholipids tend to be more enriched?
Inner leaflet of plasma membrane facing cytosol
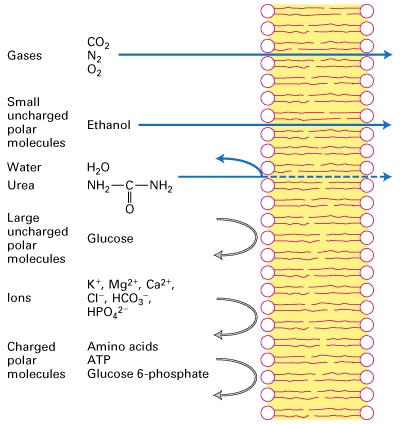
What can pass through membrane?
Gases
CO2
N2
O2
Small uncharged polar molecules
Ethanol
Water
Urea
Large uncharged polar molecules
Glucose
Ions
K+, Mg2+, Ca2+, Cl-, HCO3-, HPO4^2-
Charges polar molecules
Amino Acids
ATP
Glucose 6-phosphate
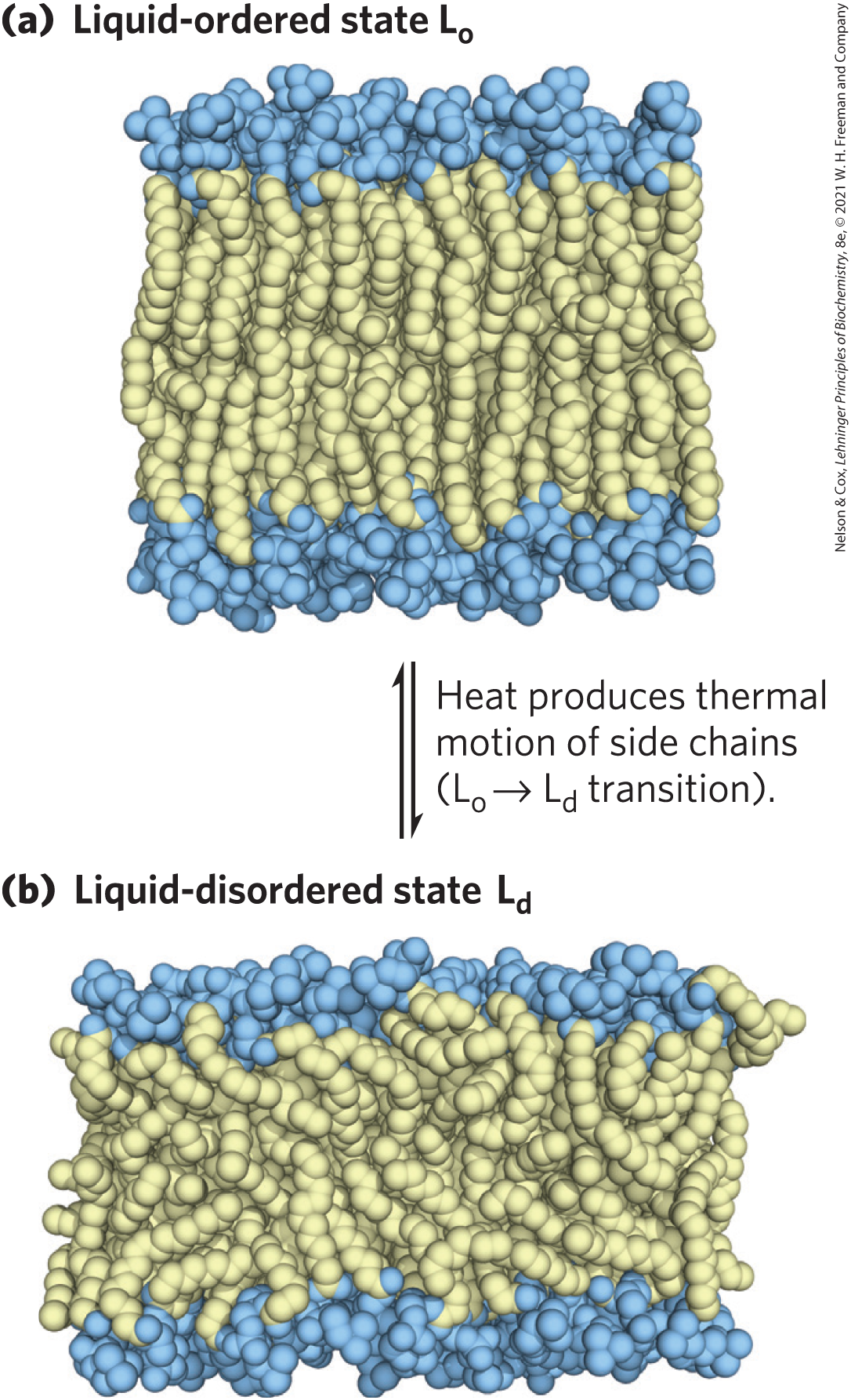
Acyl Groups in the Bilayer Interior Are Ordered to Varying Degrees
liquid-ordered (Lo) state = gel-like state in which all types of motion of individual molecules are strongly constrained
liquid-disordered (Ld) state = state in which individual hydrocarbon chains are in constant motion (lateral and rotational)
Organisms Can Adjust the Membrane Composition
Membrane fluidity is determined mainly by the fatty acid composition and melting point.
More fluid membranes require shorter and more unsaturated fatty acids.
Melting temperature decreases as double bonds are added.
Melting temperature increases with length of saturated fatty acids.
At higher temperatures, cells need more long, saturated fatty acids.
At lower temperatures, cells need more unsaturated fatty acids.
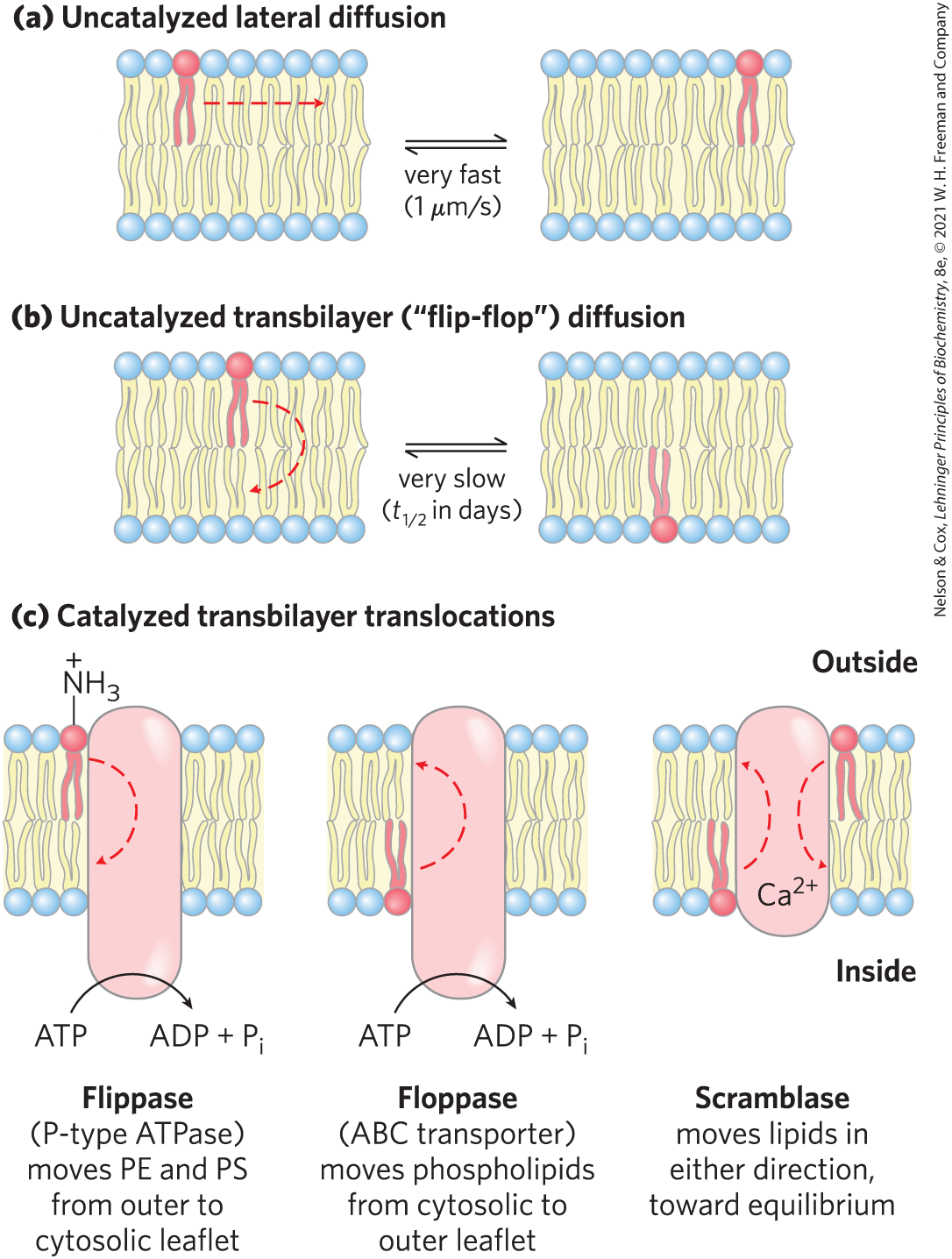
Transbilayer Movement of Lipids Requires Catalysis
Phospholipids can freely bend/flex, rotate about their axis, and laterally diffuse in bilayers
In contrast, transbilayer (“flip-flop”) movement has a large, positive free-energy change (unfavored)
membrane proteins facilitate the translocation of individual lipid molecules and maintain asymmetry
*No energy source needed = goes down its concentration gradient
*Energy source needed = goes AGAINST its concentration gradient
Functions of Proteins in Membranes
Receptors: detecting signals from outside
light (opsin)
hormones (insulin receptor)
neurotransmitters (acetylcholine receptor)
pheromones (taste and smell receptors)
Channels, carriers, transporters, pumps, flippases, etc. (nomenclature can be confusing)
nutrients (maltoporin)
ions (K-channel)
neurotransmitters (serotonin reuptake protein)
Enzymes
lipid biosynthesis (some acyltransferases)
ATP synthesis (F0F1 ATPase/ATP synthase)
proteases
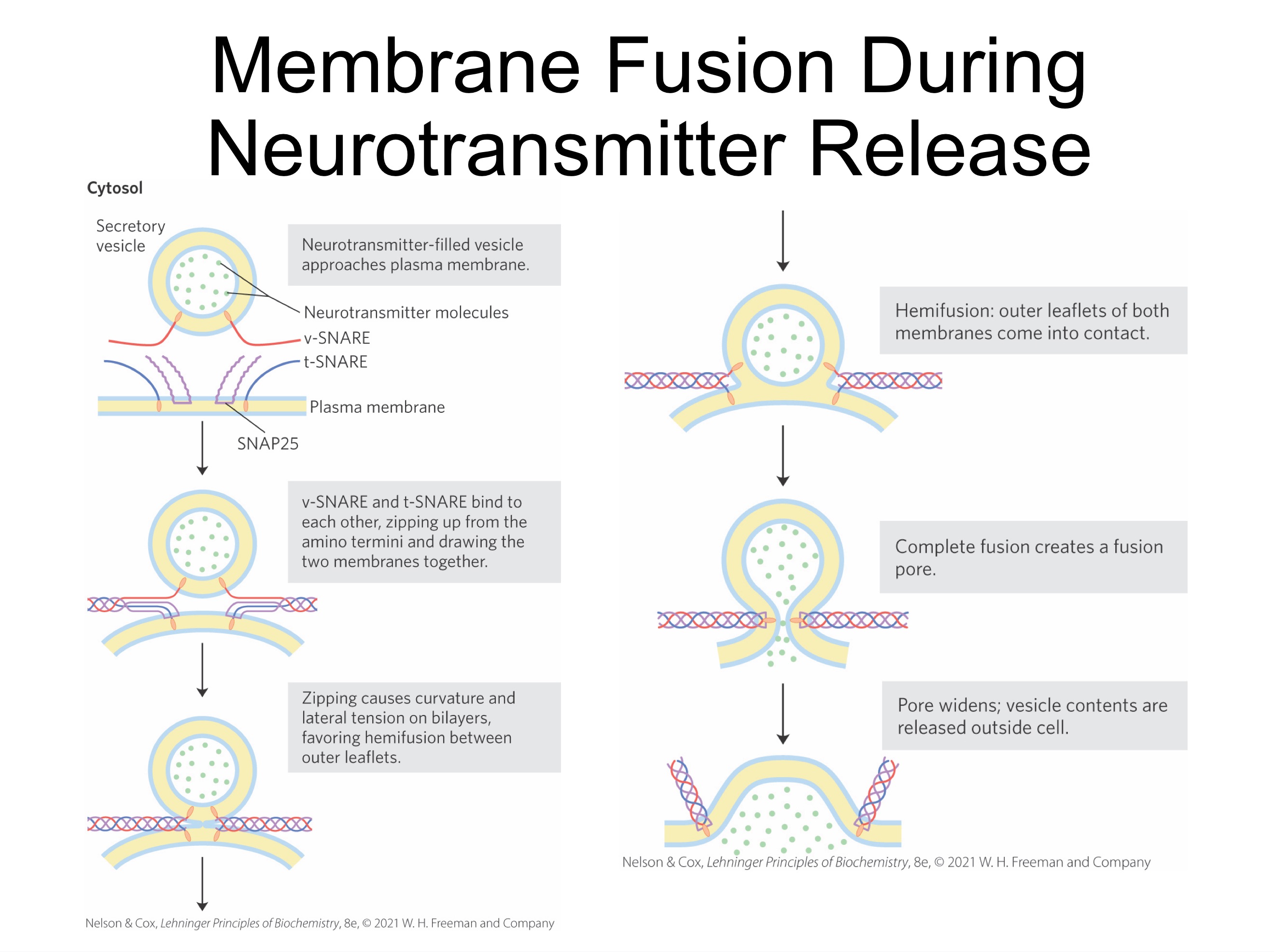
SNARE Proteins in vesicle fusion
SNAREs (snap receptors) = family of proteins
v-SNAREs = SNAREs in the cytoplasmic face of the intracellular vesicle
t-SNAREs = SNAREs in the target membrane with which the vesicle fuses
Membranes: Summary
Lipids can form micelles, bilayers, and liposomes
Membranes are composed of various lipids and proteins
Properties of the bilayer depend on the lipid composition, which varies strongly from:
organism to organism
tissue to tissue
organelle to organelle
membrane proteins are found in three major classes and play a variety of structural and functional roles, especially in the transport of solutes across the membrane
Passive transport allows passage with concentration gradient
active transport of solutes across membranes requires energy but can be accomplished in many different ways