Chapter 14 - Reactivity series
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
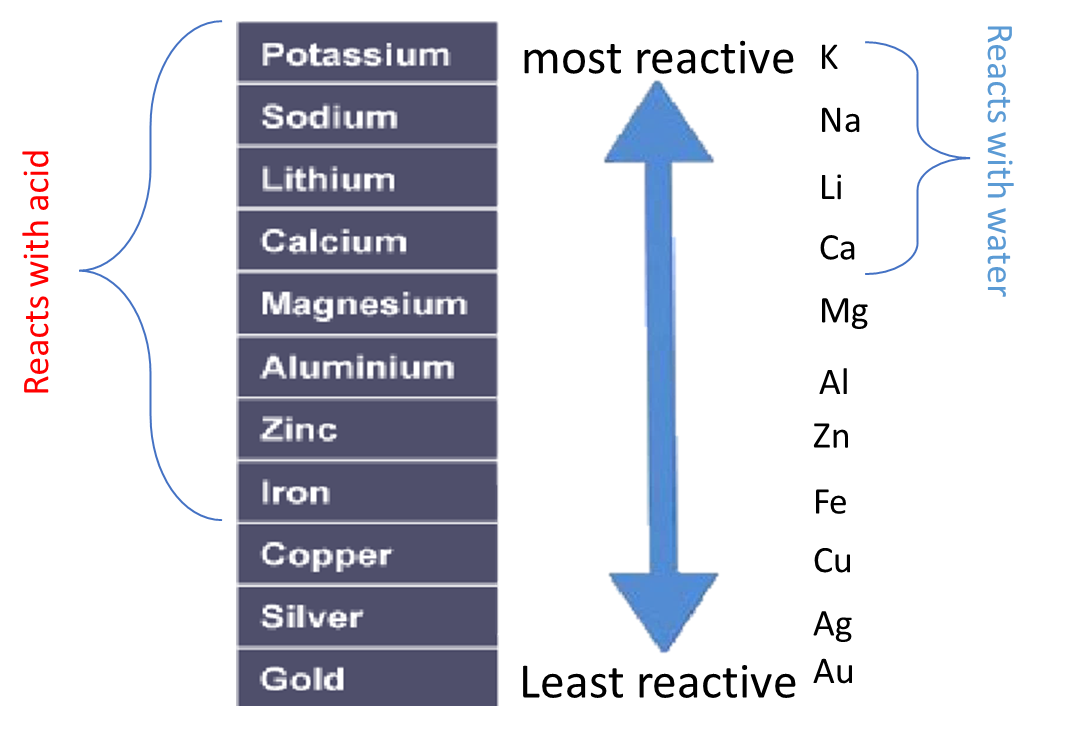
Order of reactivity and reactions with water and dilute HCl or sulfuric acid
Please Stop Calling Me American, I Like Cute Silly Goats
Some metals are more reactive than others.
The order of reactivity can be determined by adding acid to different metals and observing the rate of reaction.
For example, when hydrochloric acid is added to iron (Fe) then bubbles of hydrogen are produced slowly. However, if the same acid is added to zinc (Zn) then bubbles will be produced more quickly. This tells us that zinc is more reactive than iron.
Instead of using acid, water can be used to test the relative reactivity of metals. However, many metals are too low in the reactivity series to react with water
 Some metals are more reactive than others.
Some metals are more reactive than others.
The order of reactivity can be determined by adding acid to different metals and observing the rate of reaction.
For example, when h to react with water

understand how metals can be arranged in a reactivity series based on their displacement reactions between:
• metals and metal oxides
• metals and aqueous solutions of metal salts.
A metal will displace another metal from its oxide that is lower in the reactivity series. For example, a reaction with magnesium and copper (II) oxide will result in the magnesium displacing the copper from its oxide:
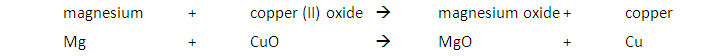
A metal will also displace another metal from its salt that is lower in the reactivity series. For example, the reaction between zinc and copper (II) sulfate solution will result in zinc displacing the copper from its salt:
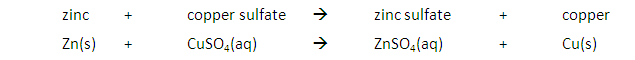
The blue colour of the copper (II) sulfate solution fades as colourless zinc sulfate solution is formed.
A more reactive metal will displace a less reactive metal:
magnesium + copper sulfate -
Mg + CuS04 -
- magnesium sulfate + copper
- MgSO4 + Cu
What are the conditions for iron to rust?
Iron rusts when oxygen and water are present.
Reaction:
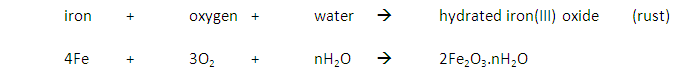
how the rusting of iron may be prevented by:
• barrier methods
• galvanising
• sacrificial protection.
Barrier Methods: Rusting may be prevented by stopping the water and oxygen getting to the iron with a barrier of grease, oil, paint or plastic.
Galvanising: (coating in zinc) also prevents water and oxygen getting to the iron, but with galvanising even if the barrier is broken the more reactive zinc corrodes before the less reactive iron. During the process, the zinc loses electrons to form zinc ions.
Sacrificial Protection: Zinc blocks are attached to iron boat hulls and underground pipelines to act as sacrificial anodes. Zinc is more reactive than iron, so oxygen in the air reacts with the zinc to form a layer of zinc oxide instead of the iron.
oxidation
Oxidation is the loss of electrons. For example a sodium atom (Na) loses an electron to become a sodium ion (Na⁺). Another example is a chloride ion (Cl⁻) losing an electron to become a chlorine atom (Cl).
Another definition of oxidation is the gain of oxygen. For example if carbon combines with oxygen to form carbon dioxide, the carbon is being oxidised.
Reduction
Reduction is the gain of electrons. For example a sodium ion (Na⁺) gains an electron to become a sodium atom (Na). Another example is a chlorine atom (Cl) gaining an electron to become a chloride ion (Cl⁻).
Another definition of reduction is the loss of oxygen. For example when aluminium oxide is broken down to produce aluminium and oxygen, the aluminium is being reduced.
Redox
A reaction involving oxidation and reduction.
A good way to remember the definitions of oxidation and reduction in terms of electrons is:
OILRIG : Oxidation Is the Loss of electrons and Reduction Is the Gain of electrons
Oxidising agent
A substance that gives oxygen or removes electrons (it is itself reduced).
Reducing
A substance that takes oxygen or gives electrons (it is itself oxidised).
2.21 practical: investigate reactions between dilute hydrochloric and sulfuric acids and metals (e.g. magnesium, zinc and iron)
Metals which are above hydrogen in the reactivity series will react with dilute hydrochloric or sulfuric acid to produce a salt and hydrogen.
metal + acid → salt + hydrogen
For example:
magnesium + hydrochloric acid → magnesium chloride + hydrogen
Mg (s) + 2HCl (aq) → MgCl₂ (aq) + H₂ (g)
This is a displacement reaction.
There is a rapid fizzing and a colourless gas is produced. This gas pops with a lighted splint, showing the gas is hydrogen.
The reaction mixture becomes warm as heat is produced (exothermic).
The magnesium disappears to leave a colourless solution of magnesium chloride.
If more reactive metals are used instead of magnesium the reaction will be faster so the fizzing will be more vigorous and more heat will be produced.
