Organic Chemistry - Nomenclature, conventions, and rules
__Naming organic molecules – nomenclature rules:
__NO. of carbons in the longest chain containing the functional group
1 = ==meth-
==2 = ==eth-
==3 = ==prop-
==4 = ==but-
==5 = ==pent-
==6 = ==hex-
==7 = ==hept-
==8 = ==oct-
- ==Count along the carbon chain such that the functional group has the lowest number
Functional groups (a prefix or a suffix)
Alkanes = ==-ane==Alkenes = ==-ene==Halogenoalkanes = ==bromo-==, ==chloro-==, etc.Alcohols = ==-anol== or ==hydroxy-
==Nitrile = ==-onitrile
==Ketones = ==-anone
==Carboxylic acids = ==-anoic acid
==Esters: alcohol name first, acid name second e.g., methyl ethanoate
Side/branched chains: Use NO. of carbons, + suffix “-yl”
CH3 = ==methyl
==CH3CH2 = ==ethyl
==CH3CH2CH2 = ==propyl
==Position of functional group or side chain:
- The number needs to indicate which carbon the group is on.
- The number is placed before the functional group name.
- The lowest number is used if there is a choice. If more than one group: use the lowest combination of numbers.
- Carboxylic acids and aldehydes are always on the “first” carbon.
More than one of the same things?
==2 = di====3 = tri====4 = tetra==Commas and dashes: a comma is put between numbers and dashes go between numbers and words. Nothing is needed between words
__Writing Formula:
__When writing the structural formula, use brackets to show when a functional group is branched off
e.g., CH3CH(CH3)CH2CH3
- (brackets show side-chain)
Skeletal formula: only shows the bonds between carbon atoms (looks like a stick figure)
Molecular formula: the exact amount of each atom in the molecule
Empirical formula: the simplest ratio of the amount of each atom in the molecule
__Conventions in Organic Chemistry:
__Curly arrow: 
- Movement of a single electron:
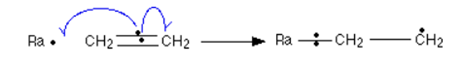
- Movement of an electron pair:
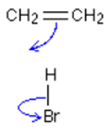 then…
then… 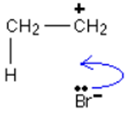
__Terminology of carbon-containing functional groups:
- __Primary carbons – carbons bonded to one other carbon
- Secondary carbons – carbons bonded to two other carbons
- Tertiary carbons – carbons bonded to three other carbons
- Quaternary carbons – carbons bonded to 4 other carbons