Alkyne Reaction and Free Radical Halogenation Reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
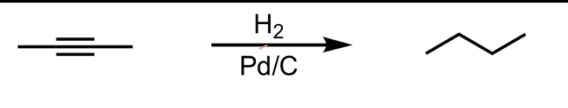
Catalytic Hydrogenation (Catalytic Reduction)
What’s added: 4 H atoms
Stereoselectivity: Anti
Rearrangement: Not possible
Note: You may see Pt used as well. This is just the catalyst and does not change the outcome of the products.
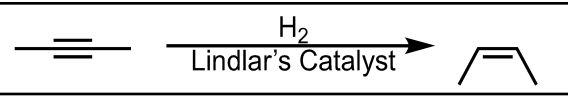
Reduction to Cis-Alkene
What’s added: 2 H atoms
Stereoselectivity: Syn
Rearrangement: Not possible
Reduction to Trans-Alkene
What’s added: 2 H atoms
Stereoselectivity: Anti
Rearrangement: Not possible
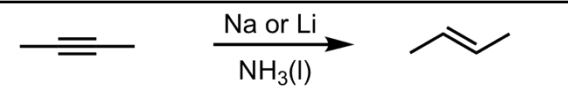
Hydrohalogenation with HBr (Terminal Alkyne)
What’s added: 1 H atom and 1 halogen atom (can be F, Br, I, or Cl) per equivalent of HX
Regioselectivity: Markovnikov
Intermediate: Carbocation
Rearrangement: Not possible
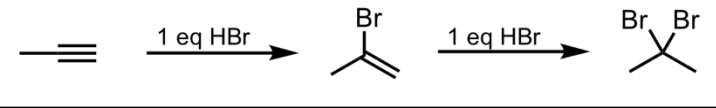
Hydrohalogenation with HBr (Internal Alkyne)
What’s added: 1 H atom and 1 halogen atom (can be Cl or Br) per equivalent of HX
Regioselectivity: Markovnikov
Intermediate: Carbocation
Rearrangement: Not possible

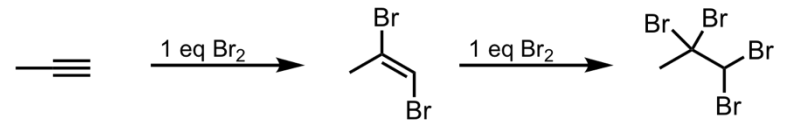
Halogenation with Br2
What’s added: 2 halogen atoms (can be F, Br, I, or Cl) per equivalent of X2
Stereoselectivity: Anti
Intermediate: Bromonium ion
Rearrangement: Not possible
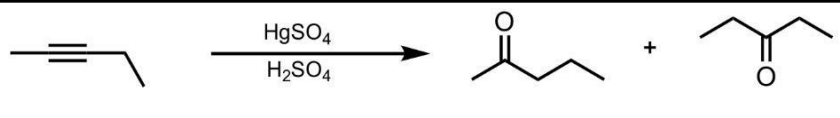
Hydration of an Internal Alkyne
What’s added: 1 O atom
Rearrangement: Not possible
Do know that this reaction produces enols, which then tautomerize to form ketones.
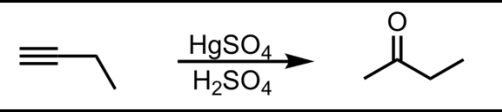
Hydration of a Terminal Alkyne (Markovnikov)
What’s added: 1 O atom
Regioselectivity: Markovnikov
Rearrangement: Not possible
Hydration of a Terminal Alkyne (Anti-Markovnikov)
What’s added: 1 O atom
Regioselectivity: Anti-Markovnikov
Rearrangement: Not possible
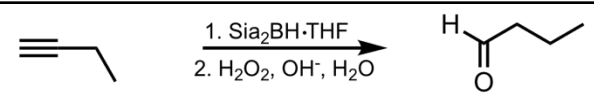
SN2 Addition of an Acetylide Ion to an Alkyl Halide
What’s added: additional C atoms (-R of alkyl halide)
Intermediate: Acetylide Ion
Rearrangement: Not possible
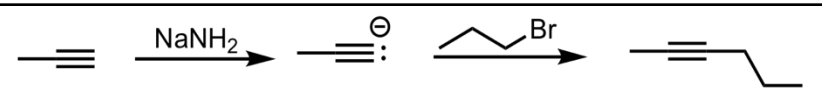
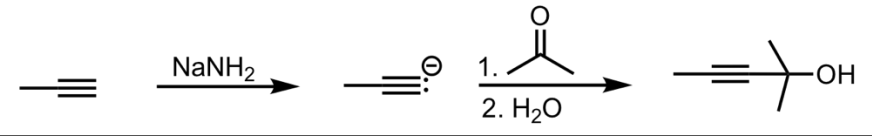
SN2 Addition of an Acetylide Ion to a Ketone
What’s added: 1 alkyl group
Intermediate: Acetylide Ion
Rearrangement: Not possible
SN2 Addition of an Acetylide Ion to an Epoxide
What’s added: 2-hydroxylpropane (from epoxide)
Intermediate: Acetylide Ion
Rearrangement: Not possible
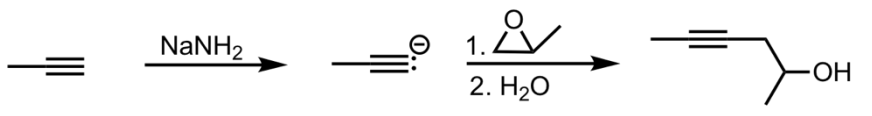
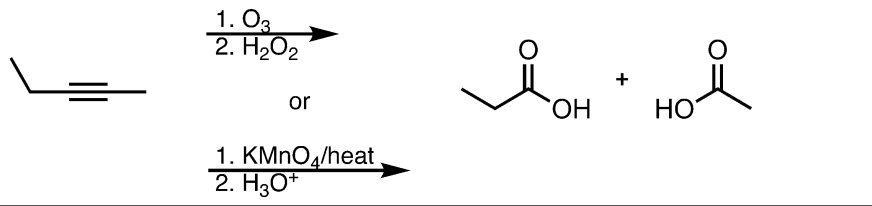
Ozonolysis/Oxidative Cleavage on an Internal Alkyne
What’s added: 4 O atoms and 2 H atoms
Know that the reaction cuts the triple bond in half. An O replaces two of the bonds as C=O and the third lone bond becomes a bond to -OH.
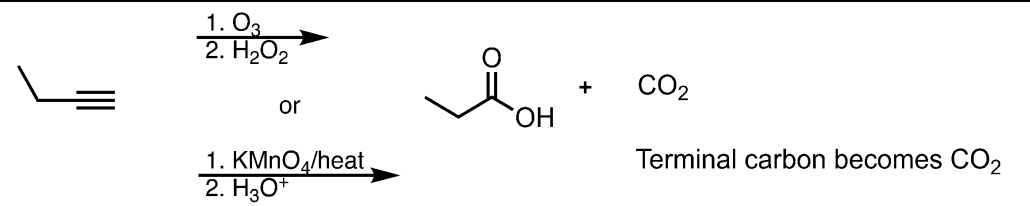
Ozonolysis/Oxidative Cleavage on a Terminal Alkyne
What’s added: 4 O atoms and 1 H atom
Know that the reaction cuts the triple bond in half. On the internal side, an O replaces two of the bonds as C=O and the third lone bond becomes a bond to -OH. On the terminal side, two oxygens O replace all the bonds on carbon, forming the most oxidized form of carbon: CO2.
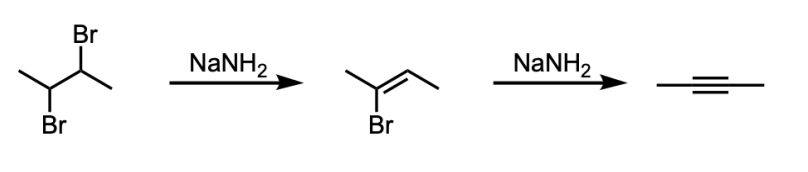
Alkyne Formation from Double Elimination of a Vicinal Dihalide
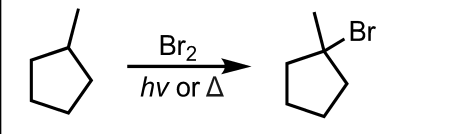
Free Radical Halogenation using Bromine (more selective)
What’s added: 1 Br atom
Regioselectivity: Most Substituted Product
Intermediate: Radical Intermediate
Rearrangement: Not possible
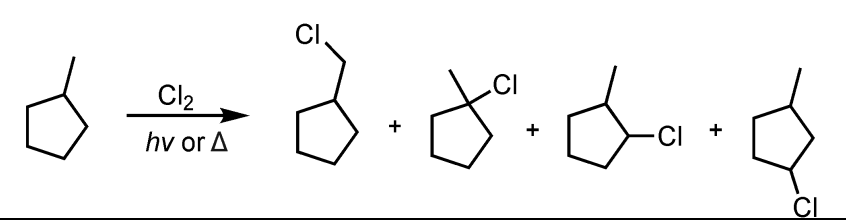
Free Radical Halogenation using Chlorine (less selective)
What’s added: 1 Cl atom
Intermediate: Radical Intermediate
Rearrangement: Not possible
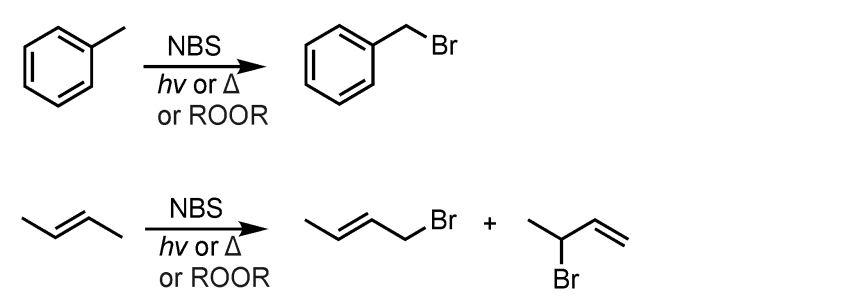
Allylic/Benzylic Bromination
What’s added: 1 Br atom
Intermediate: Allylic Radical Intermediate
Rearrangement: Not possible
Note: this reaction results in the formation of allylic radical intermediates which resonate and thus allow for the formation of multiple products.