Chemistry - Elements ,Compounds & Mixtures
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
What is an element?
Elements make up everything and are the building blocks of other substances. They consist of only ONE TYPE of atom.
Give an example of an element
Iron. If iron is melted it produces molten iron - doesn't break down into any other substance because elements are the baseline.
What are compounds?
two or more DIFFERENT types of atom that are held together by chemical bonds
Give an example of a compound
Carbon Dioxide. CO2 is one carbon atom reacting with two oxygen atoms to form a molecule of Carbon Dioxide.
What are mixtures?
two or more DIFFERENT types of atoms that are NOT chemically joined. Therefore these can be separated using physical
Give an example of a mixture
Sand and water. The two are not chemically combined and can be separated by physical means.
What does soluble and insoluble mean?
Soluble = A solid that can be dissolved in liquid.
Insoluble= A solid that cannot be dissolved in liquid.
In what situation would you use evaporation to separate a mixture?
To separate soluble solids from solutions.
Describe the steps needed in evaporation
1. Pour the solution into an evaporating dish
2. Heat the solution - the solvent will evaporate and the solution will become more concentrated. Crystals will form.
3. Keep heating until all that is left is the dry solid.
In what situation would you use filtration to separate a mixture?
To separate an insoluble solid from a liquid.
Describe the steps needed in filtration
1. Put some filter paper into a funnel and pour in your mixture.
2. The liquid will run through the paper, leaving behind solid residue.
In what situation would you use distillation to separate a mixture?
To separate a liquid from a solution.
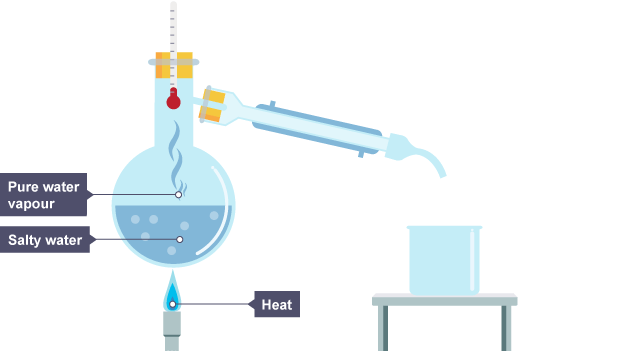
Describe the steps needed in distillation
1. Heat the solution - this will cause the part of the solution with the lowest boiling point to evaporate.
2. This vapour rises, and passes through a tube surrounded by cold water - this cools and condenses the vapour and it becomes liquid.
3. The liquid then runs down the tube into a separate flask.
What is chromatography?
The separation of a mixture by passing it through a medium in which the components move at different rates.
You only need to understand Paper Chromatography! Describe this process
1. Take a piece of filter paper and, using a pencil, draw a line near the bottom of it.
2. Add spots of different ink on the line at regular intervals.
3. Loosely roll up the sheet and place it in a beaker of water - use other liquid if needed.
4.make sure the water is just below the baseline drawn
5. Place a lid on top and wait.
6. The solvent will seep up the paper, taking the inks with it - each ink will move up the paper at a different rate and end in a different spot.
7. take the paper out and let it dry.
How does chromatography separate the mixtures?
Some dyes will be more attracted to the paper and spend more time stuck to it (less soluble) not travelling up it. Others will be more soluble in the solvent and will travel further up the paper
Why would someone use chromatography?
To see if a substance is present in a mixture. You would run a pure sample of the substance alongside the mixture - if the two have the same Rf value the substance is likely present in the mixture.
What is Rf value and how do you calculate it?
Rf value is the ratio between the distance the dissolved ink travelled, and the distance the solvent used travelled. Rf = distance moved by ink / distance moved by solvent front.
What is a molecule?
A group of two or more atoms held together by chemical bonds (can contain different atoms)
What is a solution?
formed when one substance (solute) dissolves in another substance (solvent)
What happens when you use a pure substance for chromatography?
If a pure substance was used (substance with only 1 type of chemical) the substance wouldn’t separate but instead a single spot would appear on the paper
What factors affect the Rf value??
type of paper
type of solvent
if a pure substance was used