physics - energy test
1/22
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
what are the 8 energy stores?
kinetic
thermal
magnetic
gravitational potential
elastic
electrostatic
nuclear
chemical
what are the 5 ways energy can be transferred?
mechanically
electrically
heat (by conduction, convection or radiation)
radiation (by light or sound)
what does dissipated energy mean?
when energy is transferred to the surroundings and is difficult to recover.
what is the principle of conservation of energy?
energy cannot be created or destroyed, only transferre
what is energy efficiency?
when an object uses less energy to perform the same task, and does not waste much of it’s input energy
how do you claculate energy efficiency
useful output energy / total input energy x 100
in a sankey diagram, does the length or the width of the arrows matter?
the width
explain the energy transfers when a cyclist rides a bike up a hill
bottom of the hill: maximum kinetic energy and minimum gravitational energy
moving up the hill: gain gravitational potential energy as they get higher, and lose kinetic energy as they slow down.
top of the hill: have the maximum gravitational energy and the minimum kinetic energy
down the hill: lose gravitational energy and gain kinetic energy
the whole time they lose chemical energy as they burn the energy their food is giving them to help them move.
explain the energy transfer as a boat accelerates
chemical energy (fuel) > kinetic energy (gaining speed)
thermal energy transfer
how can thermal energy be transferred?
conduction
convection
radiation
how does conduction work
it occurs mainly in solids
the particles closest to the heat source heat up, mening they gain kinetic energy
as they gain kinetic energy, they vibrate faster
this means they collide with the particles surrounding them
these particles then vibrate more
this continues, transferring the thermal energy (particles gain kinetic energy as they heat up)
how does convection work?
only occurs in fluids
the particles closest to the heat source heat up and gain kinetic energy
they spread apart, meaning they become less dense
as they become less dense they rise, leaving a gap where the particles previously were
they are replaced by cooler, more dense particles
as the hotter particles rise, they colid with other particles in the surroundings and lose their kinetic energy (they become cooler and more dense)
they sink as the newly heated particles rise, forminag a convection current
how does radiation work?
requires no particles
energy is transferred by infrared waves
this is why we can feel the suns heat through the vacuum of space
the hotter an object is, the more radiation it emits.
Dull and dark surfaces are poor reflectors but good absorbers. light and shiny surfaces are good reflectors, but poor absorbers.
examples of convection in everyday phenomena
radiators:
the air molecules closest to the radiator heat up, become less dense and rise
these heated particles spread throughout the room and are replaced by cooler, less dense particles
these particles heat up and rise, while the previously heated particles cool (collide with other particles and lose kinetic energy), and sink
this sets up a convection current
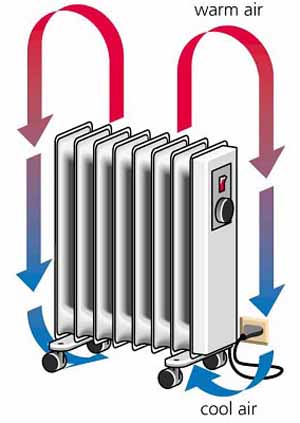
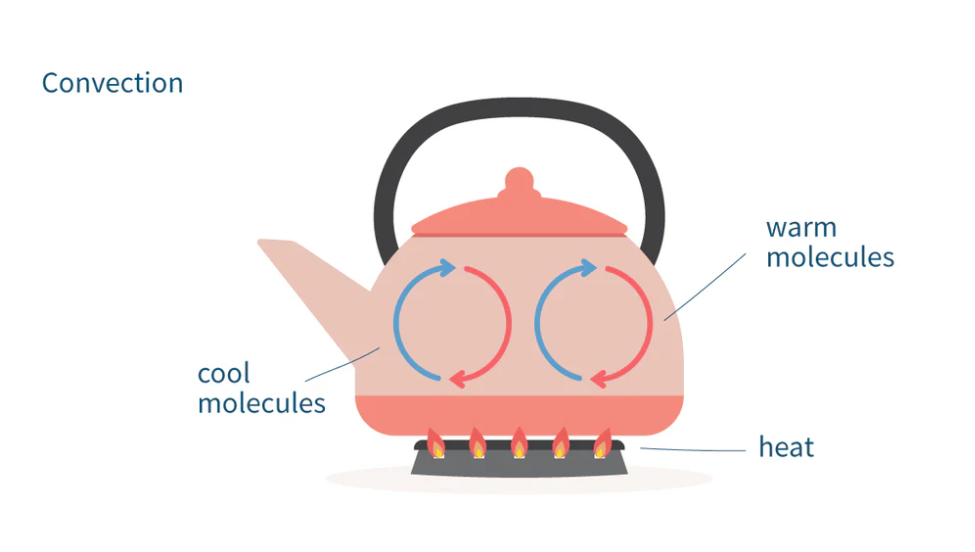
examples of convection in everyday phenomena
boiling kettle:
the water molecules at the bottom of the kettle heat up first and rise, and are replaced with cooler molecules
these cooler molecules heat up and rise
this continues until the all the water in the kettle is heated
how does surface and temperature relate to emission and absorption of radiation?
the hotter an object, the more radiation is gives out (emits)
if an object is a good emitter, it is also a good absorber
this means that hotter objects are good emitter and absorbers of radiation
dark, dull objects absorb and emit more radiation, while light and shiny object reflect more thermal radiation
reducing unwanted energy transfer
reducing unwanted energy transfer
lubrication:
reducing thermal energy transfer by oiling machinery paarts to reduce friction
insulation - preventing thermal energy transfer:
cavity wall insulation:
filling the space between 2 walls with a lightweight solid with pockets of air
as air is a bad conductor, it traps the heat and prevents it from escaping
as the material is a solid, it prevents heat transfer by convection (and minimises heat transfer by conduction)
double glazing:
a vacuum between 2 panes of glass
as there are no particles, it prevents thermal energy transfer by conduction and convection
Loft insulation:
same as cavity wall insulation but on the roofs of lofts (as hotter air rises upwards, it prevents the ehat from escaping the house)
painting houses white in hot countries:
as white is a good reflector of thermal radiation, houses in hotter countries stay cool
practicals
conduction practical
vaseline pins:
attatch drawing pins to the end of identical rods made from different materials with vaseline
heat up the rods
the thermal energy transfers from one end of the rod to the other via conduction, as the particles collide and transfer the vibrations (as they collide it creates friction, which generates heat energy which is transferred)
eventually the thermal energy is transferred to the end of the rod
the faster the drawing pins fall of the ends of the rods, the better conductor of heat the material the rod is made out of is
convection practical
smoke chimney:
a candle is placed under the right hand chimney
it heats up the air molecules surrounding it
they become less dense and rise, leaving a gap there they previously were
air rushes down through the left hand chimney to fill the gap
these new particles heat, while the previously heated ones collide with other particles, losing their kinetic energy (how they store thermal energy) and sink
this creates a convection current
radiation practical
Leslie’s cube:
4 faces are all different (dark matte, dark shiny, light matte, light shiny)
fill the cube with hot water
leave it for one minute, so the surfaces can heat up
used an infrared detector to measure the temperature of each face
Observations:
The dark, matte surface will emit the most infrared radiation.
The light, shiny surface will emit the least infrared radiation.
because good emitters are also good absorbers, dark matte objects are the best absorbers and emitter sof thermal radiation, light shiny objects are the worst (best reflectors)