Acid/base balance: acidosis and alkalosis
1/12
Earn XP
Description and Tags
lesson 4, week 4, unit 5
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
bicarbonate as a buffer
it will prevent the pH from rising too quickly or dropping too quickly
what happens when the blood is more basic
the kidneys will conserve protons and may excrete bicarbonate. when the blood first filters into the corpsuscle into the bowmanns space, bicarbonate is easily filtered in that solution. the kidneys will participate in acid base balance by reabsorbing almost all of that bicarbonate right away, regardless of the pH of the blood. reabsorption of bicarbonate will occur in the proximal tubule and the conservation of the buffering system. in the collecting duct, 70% are principal cells and the rest are intercalated cells (type a and b). type are involved in secretion of protons and therefore excreting protons from the blood. type b are involved in secretion and excretion of bicarbonate
Therefore, the kidneys will:
Conserve filtered bicarbonate (HCO3-), the main buffer of the blood
Excrete hydrogen ions (H+ ): blood pH is too acidic
Excrete HCO3- : blood pH is too alkaline
bicarbonate in the tubules (conserving bicarbonate)
bicarbonate is present in the blood, easily filtered, and is present in the tubule. with the sodium and h exchanger, h is also present in the tubule and together, they make co2 and water. water can easily travel across the luminal membrane into these cells bc of the water channels. co2 doesnt need help bc its small. co2 and water combines in the presence of carbonic anhydrase to form h and bicarbonate. we have a protein carrier in the basolater membrane that transports bicarbonate into the surrounding interstitium and then into the blood (easily reabsorbed). no bicarbonate transporter so longer process
type a and type b intercalated cells
When blood pH is more acidic, type A intercalated cells will be activated to secrete H+ into the tubule lumen. Secretion of H+ results in excretion in the urine, reducing the amount of H+ ions that were in the blood.
Alternatively, when the blood pH is more alkaline, type B intercalated cells will be activated instead to secrete HCO3- in the tubule lumen, which will then be excreted in urine. Removing HCO3- from the blood will assist with reducing the blood pH, making it less alkaline.
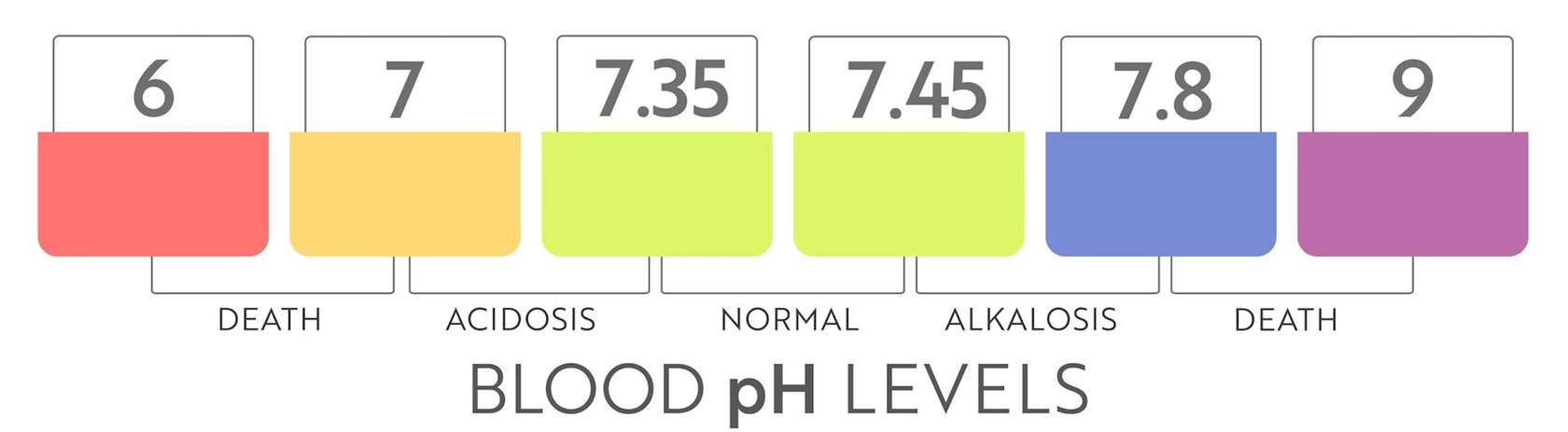
what is our blood pH level range
Normal blood pH is around 7.4. If blood pH drops below that, it is considered acidosis (blood too acidic). It is considered alkalosis if it rises above 7.4 (blood too alkaline). Extreme changes in blood pH can result in death.
What can cause acidosis or alkalosis?
Respiratory acidosis and respiratory alkalosis: pH problems that arise because of changes to lung function related to alveolar gas exchange.Metabolic acidosis and metabolic alkalosis: pH problems that arise unrelated to the lungs. This could be from kidney issues or a host of other conditions. We call these metabolic causes.
Respiratory acidosis: Blood pH less than 7.4
when PCO2 increases, H+ levels also increase in the blood due to the production of H+ during the bicarbonate reaction. This means that any lung conditions that prevent CO2 from being properly exhaled from the alveoli can result in respiratory acidosis. This includes: Hypoventilation, Pulmonary fibrosis, Emphysema
Hypoventilation:
underventilating the alveoli. Some medications can cause people to breathe shallowly or slowly, reducing alveolar ventilation.
Pulmonary fibrosis:
we learned that in this lung disease, the alveolar walls thicken and develop scar tissue. This causes poor gas exchange and therefore CO2 does not readily leave the blood at the pulmonary capillaries to be exhaled out.
Emphysema:
alveolar walls begin to break down in this lung disease, reducing surface area, which hinders appropriate gas exchange.
Respiratory alkalosis: Blood pH higher than 7.4
If PCO2 is too low, we make less H+ since the bicarbonate reaction is not occurring. This leads to a higher blood pH. Any lung conditions that would overventilate the alveoli, exhaling out excess CO2 can cause respiratory alkalosis. Hyperventilation can lead to this condition.
reasons a person might hyperventilate
This disruption in normal PCO2 and pH can affect multiple body systems. For example, we discussed how low pH and high PCO2 act as vasodilator metabolites (VDMs) in the cardiovascular unit, leading to vasodilation of arterioles. In hyperventilation, we actually trigger vasoconstriction due to the reduction in these VDMs, which may reduce blood flow to the brain and cause symptoms like dizziness or even fainting.