Trends in the Periodic Table
1/59
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
60 Terms
When atoms come together to connect, several factors are important, such as:
WHAT
WHAT
WHAT
When atoms come together to connect, several factors are important, such as:
Size
Ease of losing electrons (how tightly their valence electrons are held together)
Desire for gaining an electron or sharing an electron (depends on effective nuclear charge (Z*eff) and distance from the nucleus (shell #n))
Many properties of an atom depend on how tightly its WHAT are attracted to the WHAT, and the amount of WHAT charge that they feel
Many properties of an atom depend on how tightly its VALENCE ELECTRONS are attracted to the NUCLEUS, and the amount of POSITIVE charge that they feel
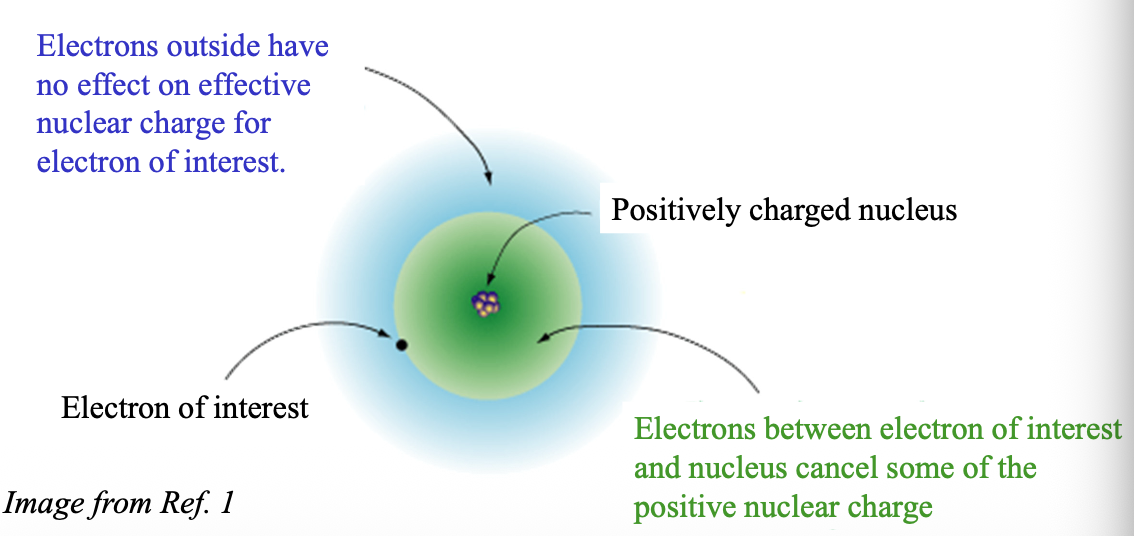
Usually, the WHAT electrons block the WHAT electrons from experiencing the full attraction of the nucleus. This is called “WHAT”
Usually, the CORE electrons block the VALENCE electrons from experiencing the full attraction of the nucleus. This is called “SHIELDING”
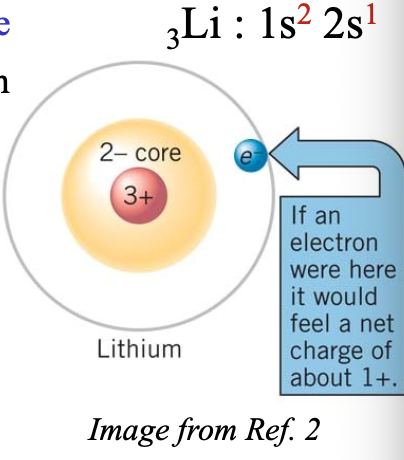
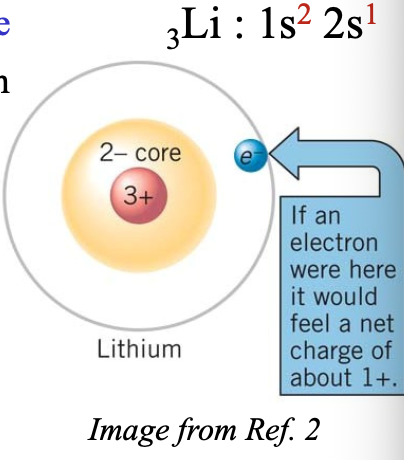
Example:
The 2s electron of Li atom only feels the net charge of WHAT
Example:
The 2s electron of Li atom only feels the net charge of +1
Effective nuclear charge (Z*eff) is the actual WHAT charge “felt” by an electron in an atom (other than hydrogen)
Effective nuclear charge (Z*eff) is the actual POSITIVE charge “felt” by an electron in an atom (other than hydrogen)
Z*eff = WHAT
Z*eff = Z - S
Z = Atomic number = nuclear charge
S = number of shielding core electrons

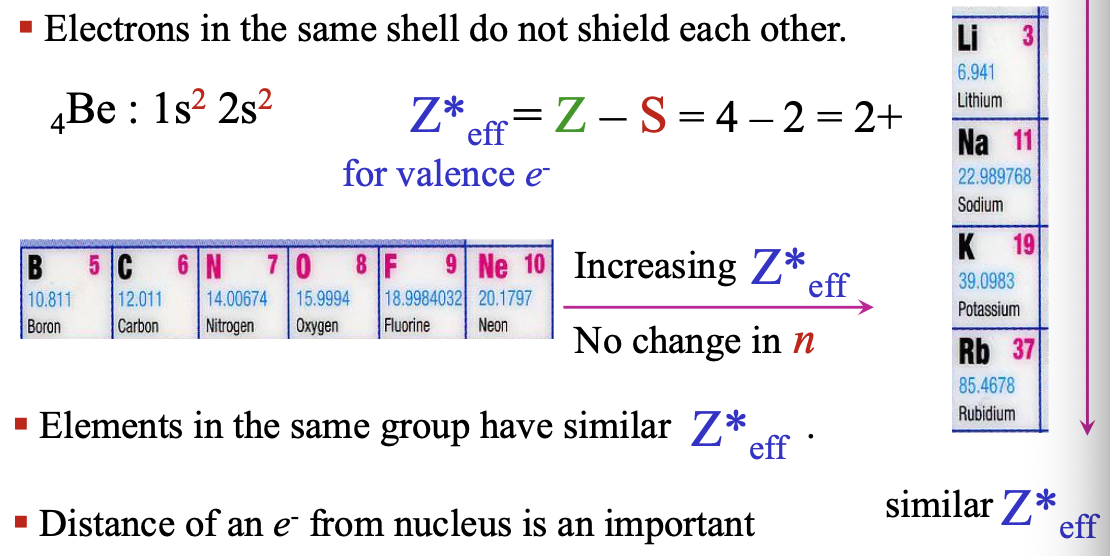
Electrons in the same shell do not WHAT each other
Electrons in the same shell do not SHIELD each other
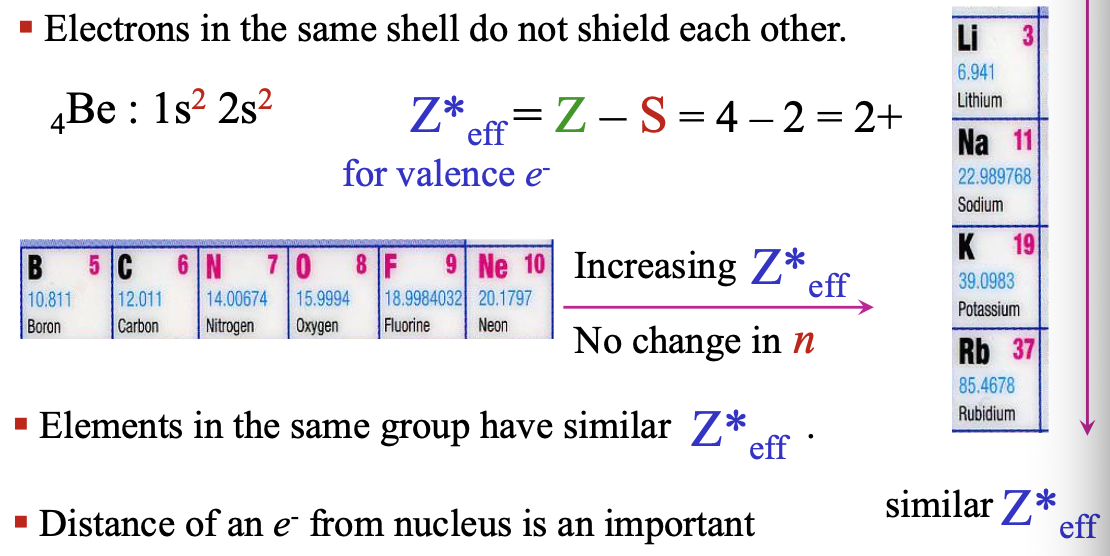
Elements in the same group have similar WHAT
Elements in the same group have similar Z*eff
The distance of an electron from the nucleus is an important factor in the “WHAT” it feels (Z*eff) from the WHAT
The distance of an electron from the nucleus is an important factor in the “PULL” it feels (Z*eff) from the POSITIVE NUCLEUS
The distance of the valence electron from the nucleus increases by increasing the WHAT
The distance of the valence electron from the nucleus increases by increasing the n
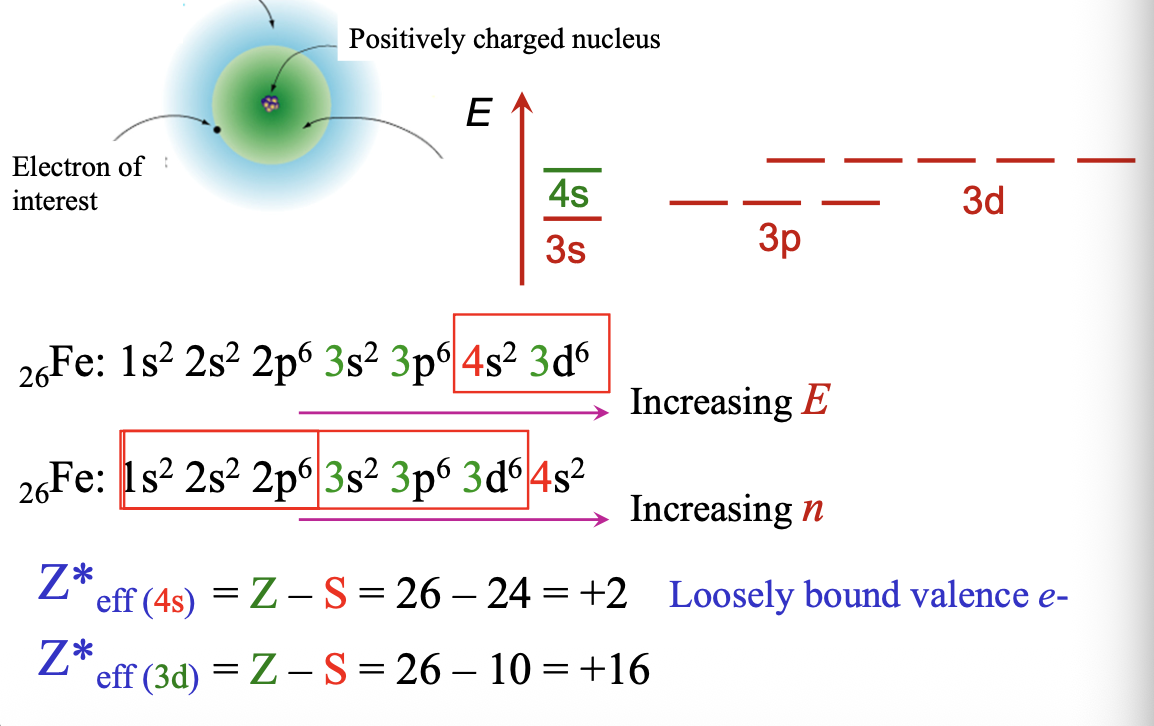
Shielding and effective nuclear charge example
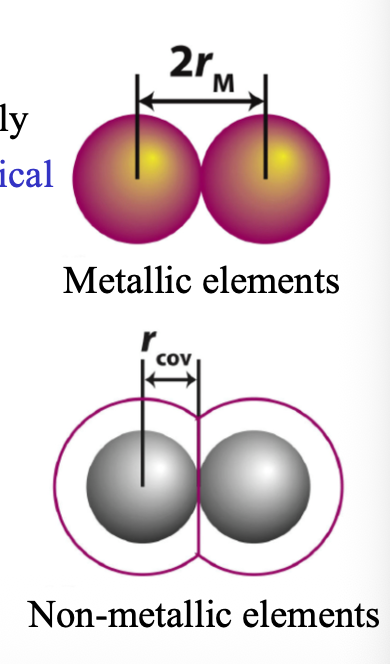
The electron cloud around nucleus has no WHAT so how do you measure the atomic radius
The electron cloud around the nucleus has no DEFINITE LIMIT/EDGE so how do you measure the atomic radius
Atomic radius is WHAT of the experimentally determined distance between two WHAT
Atomic radius is HALF of the experimentally determined distance between two IDENTICAL NEIGHBORING NUCLEI IN SOLID
Factors affecting the atomic size:
WHAT
WHAT
Factors affecting the atomic size:
Principal quantum number (n) for valence electrons
Effective nuclear charge felt by valence electrons
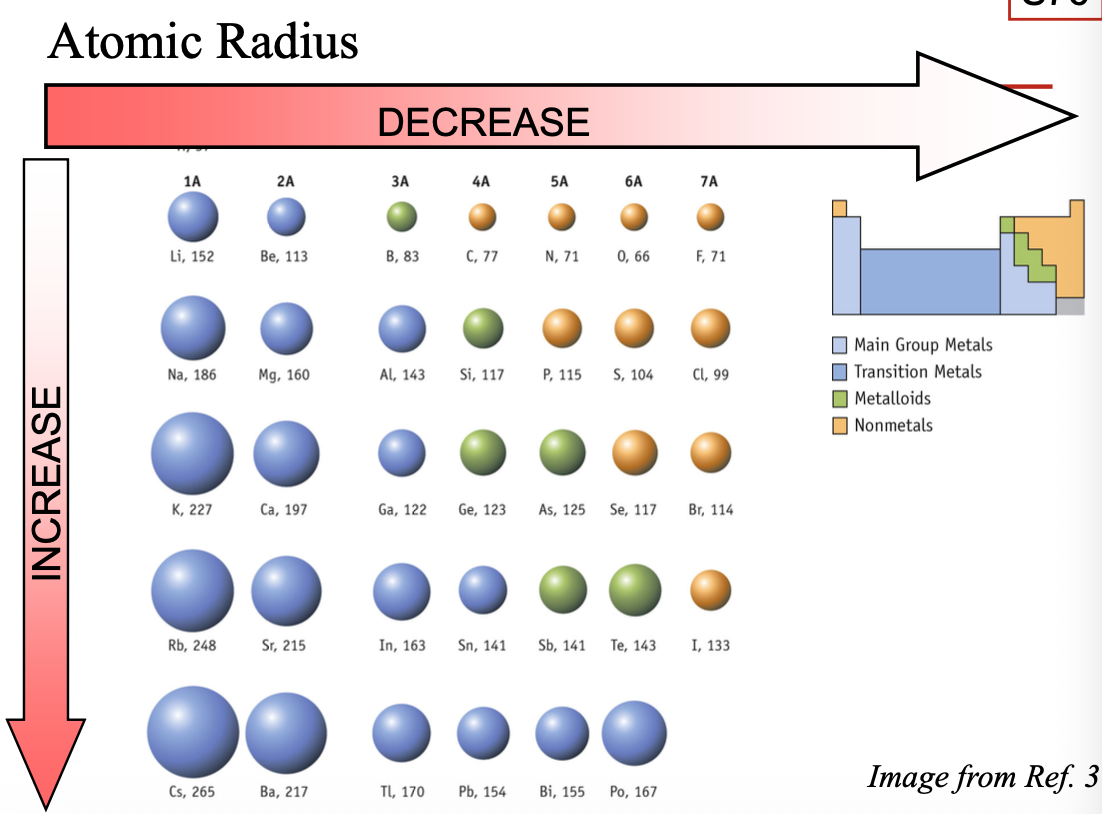
Going from top to bottom in a group
The principal quantum number (n) of the valence electrons WHAT
The effective nuclear charge for the valence electrons stays WHAT
Going from top to bottom in a group
The principal quantum number (n) of the valence electrons INCREASES
The effective nuclear charge for the valence electrons stays ALMOST STAYS THE SAME
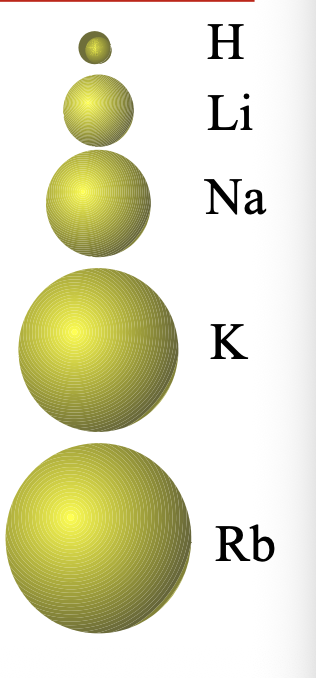
The larger the n value, the larger are the WHAT taht contain the WHAT electrons
The larger the n value, the larger are the ORBITALS taht contain the VALENCE electrons
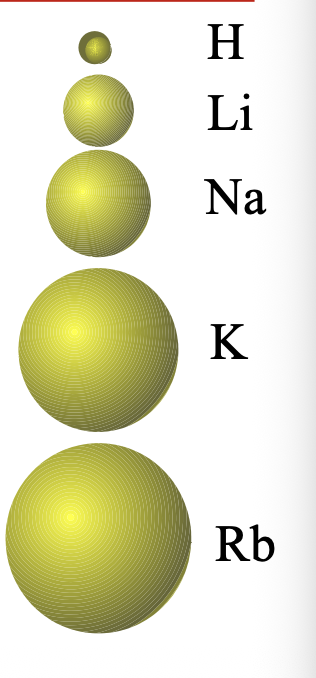
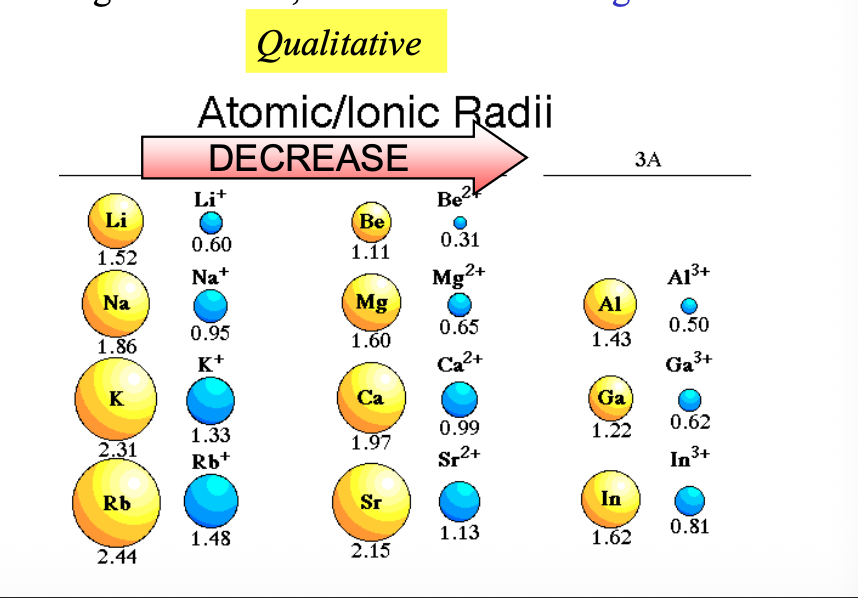
Atoms become larger by going from WHAT to WHAT in a group
Atoms become larger by going from TOP to BOTTOM in a group
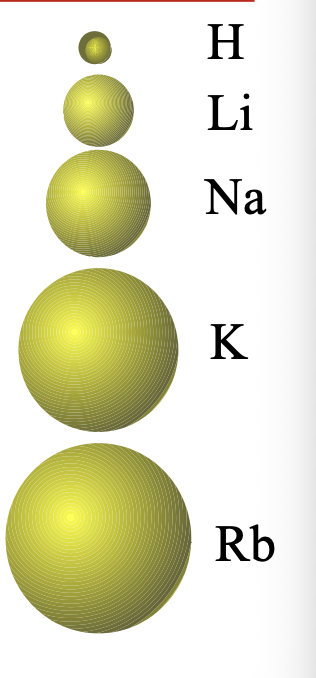
Going from left to right across a period
Adds electrons to the same WHAT
The nuclear charge WHAT, while the number of core electrons stays the WHAT; effective nuclear charge for the valence electrons WHAT
Going from left to right across a period
Adds electrons to the same SHELL (n)
The nuclear charge INCREASES, while the number of core electrons stays the SAME; effective nuclear charge for the valence electrons INCREASES
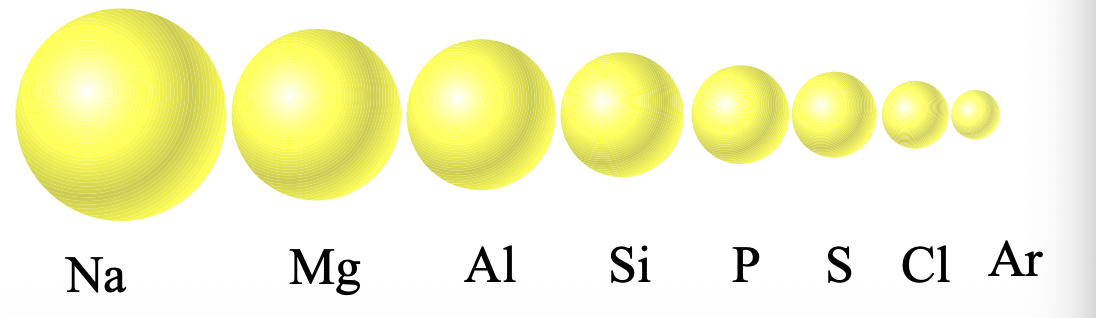
Electrons are pulled closer to the WHAT as WHAT increases
Electrons are pulled closer to the NUCLEUS as Z*eff increases
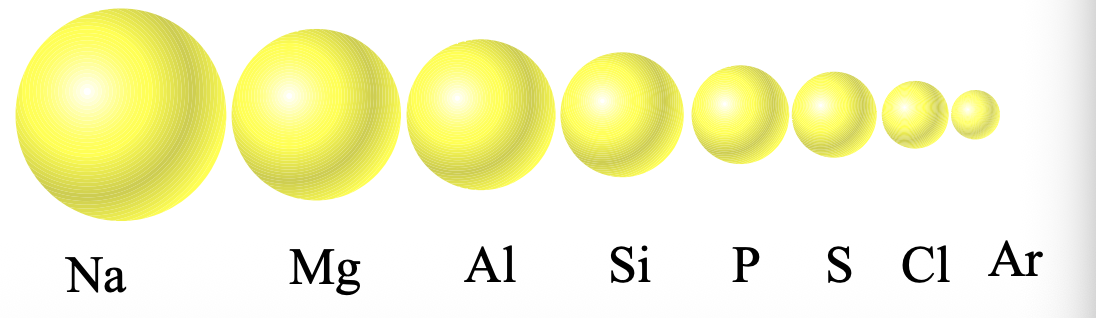
Atoms become WHAT by going left to right in a period
Atoms become SMALLER by going left to right in a period
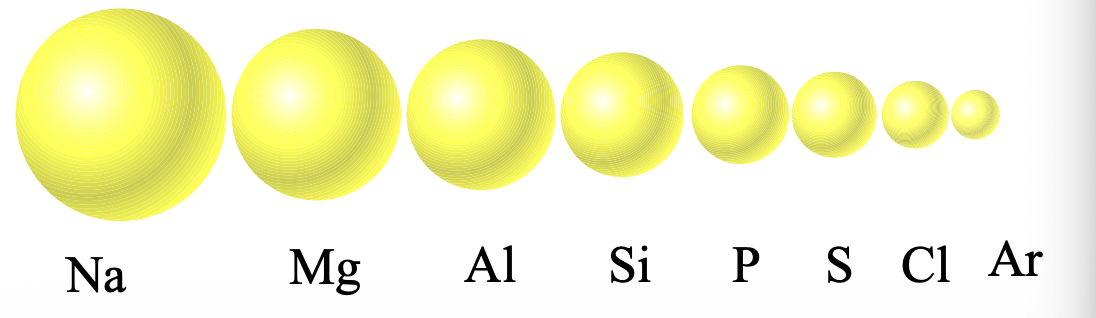
Ions are formed by adding or removing WHAT to/from the WHAT
Ions are formed by adding or removing ELECTRONS to/from the VALENCE SHELL
When sodium loses its valence electron, it still has HOW MANY protons and HOW MANY electrons left, so a positively charged particle is formed = WHAT
When sodium loses its valence electron, it still has 11 protons and 10 electrons left, so a positively charged particle is formed = CATION
Cations are always WHAT than original atoms
Cations are always SMALLER than original atoms
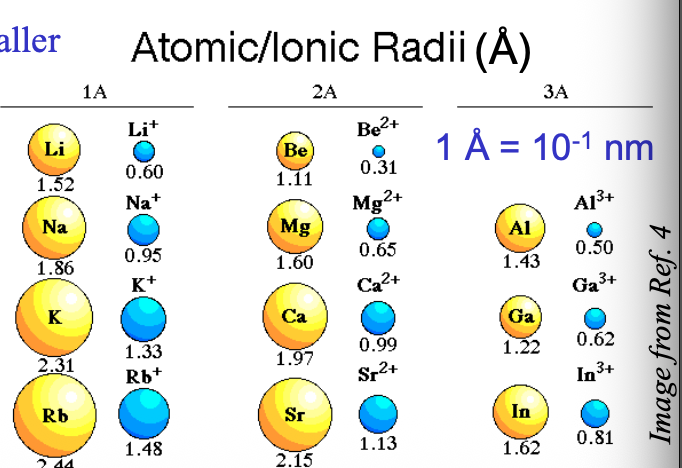
Number of protons are WHAT than the number of electrons in the cation, so the electrons are pulled WHAT to the nucleus → makes it smaller
Number of protons are MORE than the number of electrons in the cation, so the electrons are pulled CLOSER to the nucleus → makes it smaller
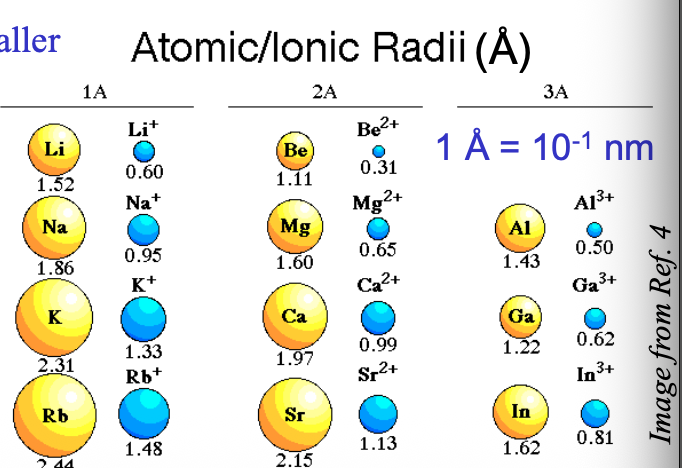
Going from top to bottom in a group, the size of cations with the same WHAT becomes WHAT, following the same trend as atom sizes
Going from top to bottom in a group, the size of cations with the same CHARGE becomes LARGER, following the same trend as atom sizes
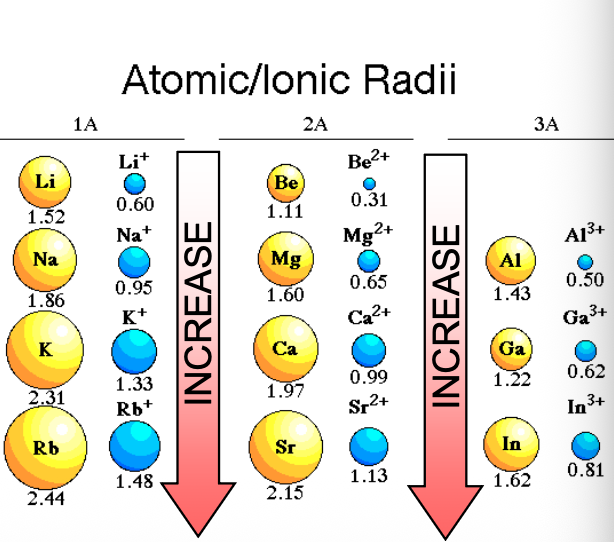
Going from left to right in a period, the size of cations with different charges WHAT as the nuclear (+) charge WHAT
Going from left to right in a period, the size of cations with different charges DECREASES as the nuclear (+) charge INCREASES
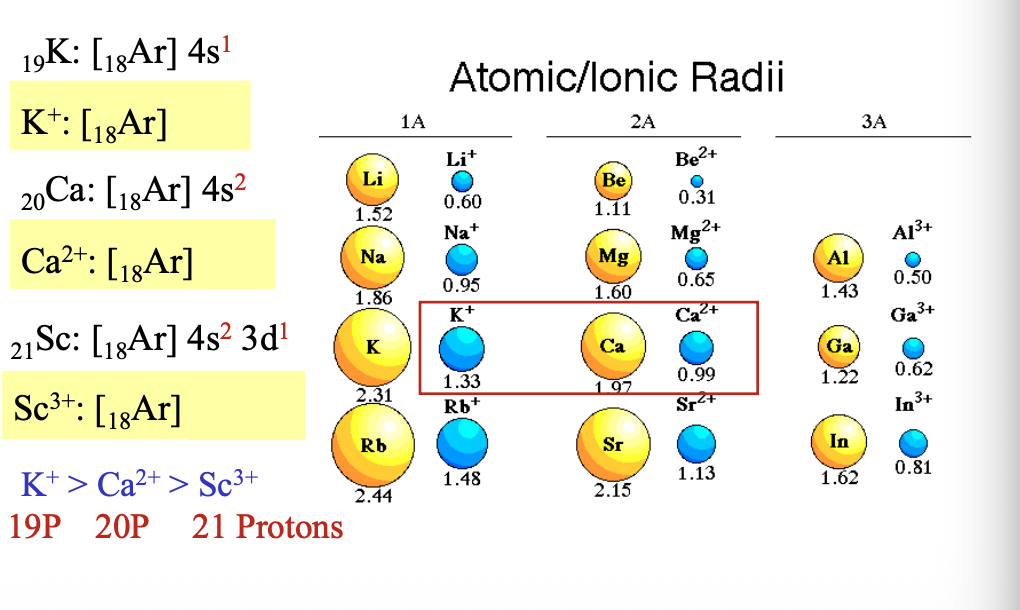
Isoelectronic species have the same WHAT
Isoelectronic species have the same NUMBER of ELECTRONS
Sometimes a metal atom can lose more than one WHAT
Sometimes a metal atom can lose more than one ELECTRON
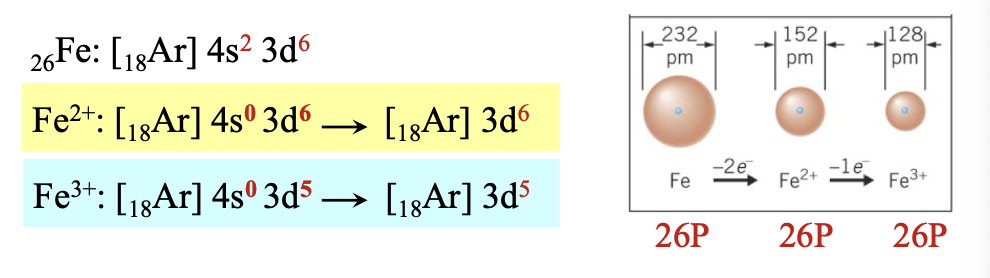
Transition metals always first lose electrons from the WHAT (ns), and then from the WHAT (n-1)d
Transition metals always first lose electrons from the OUTERMOST SHELL (ns), and then from the INNER SHELL (n-1)d
Less electrons for the same number of protons = WHAT
Less electrons for the same number of protons = STRONGER PULL for those electrons
WHAT tend to gain one or more electrons in WHAT shell to reach the electronic configuration of the noble gas next to them
NON-METALS tend to gain one or more electrons in VALENCE shell to reach the electronic configuration of the noble gas next to them
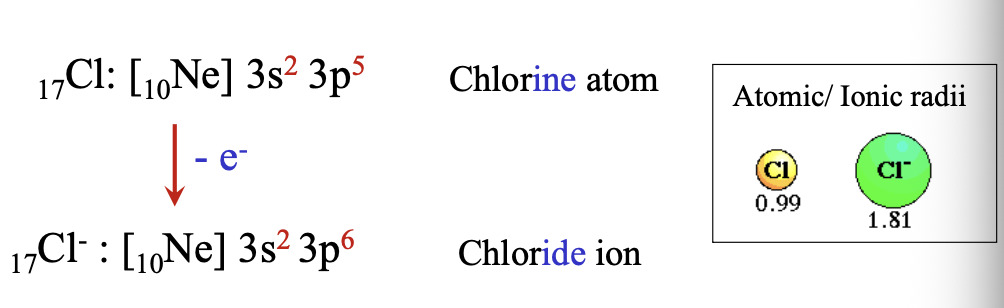
When chlorine atom gains an electron, there are 17 protons and WHAT electrons so a negatively charged particle forms a WHAT
When chlorine atom gains an electron, there are 17 protons and 18 electrons so a negatively charged particle forms a ANION
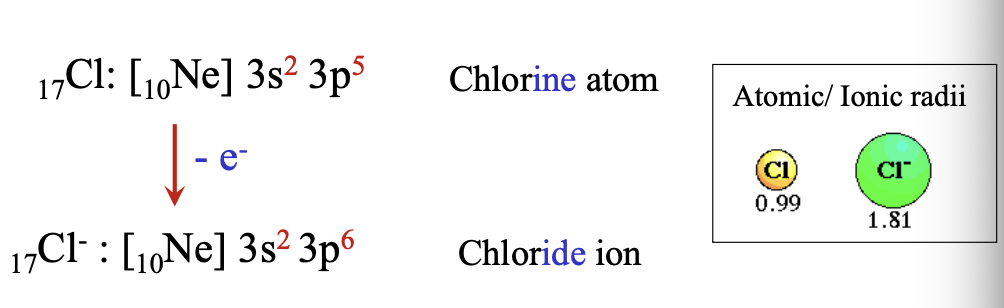
Anions are always WHAT than their parent atoms
Anions are always LARGER than their parent atoms
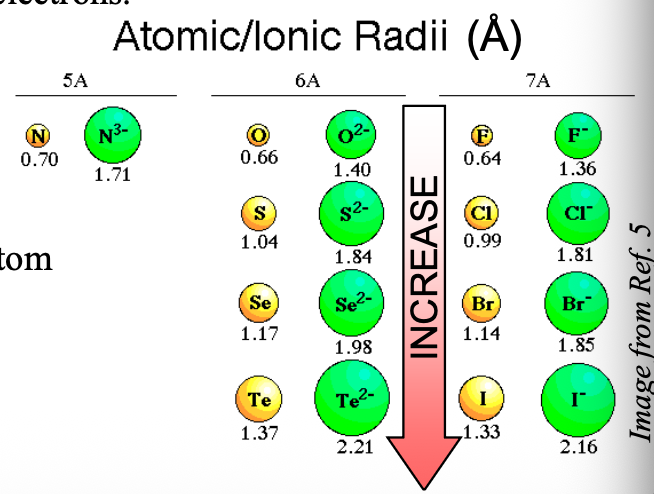
Number of protons are WHAT than number of electrons in the anion, so the electrons are WHAT pulled WHAT the nucleus. Also more WHAT between electrons
Number of protons are LESS than number of electrons in the anion, so the electrons are LESS pulled TOWARD the nucleus. Also more REPULSION between electrons
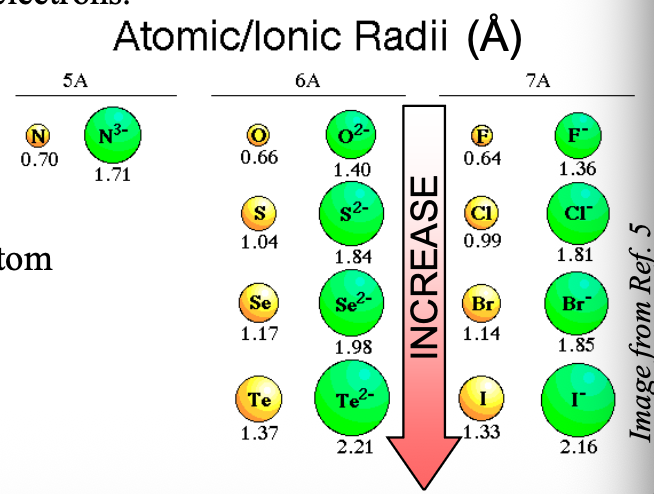
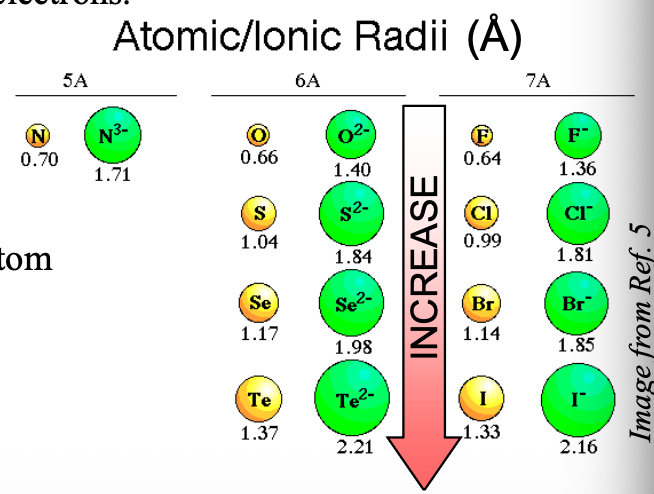
Going from top to bottom in a group, the size of anions with the same charge become WHAT
Going from top to bottom in a group, the size of anions with the same charge become LARGER
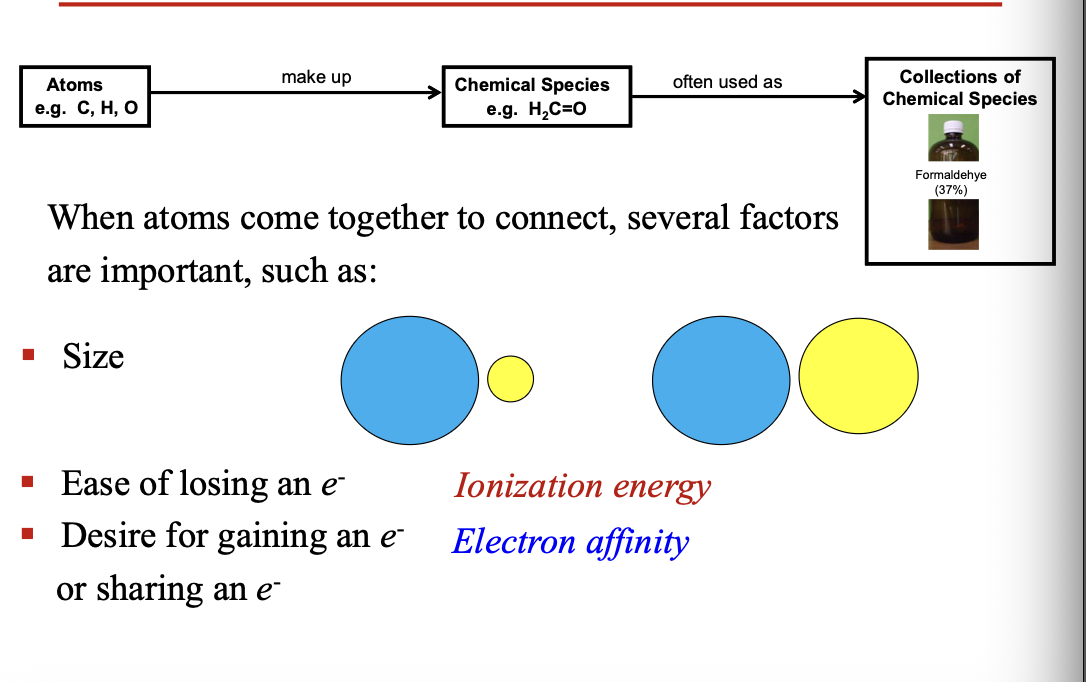
When atoms come together to connect, several factors are important
WHAT
WHAT
WHAT
When atoms come together to connect, several factors are important
Size
Ease for losing an electron = Ionization energy
Desire for gaining an electron or sharing an electron = Electron affinity
Ionization energy is the energy (kJ/mol) required to remove one WHAT from one WHAT
Ionization energy is the energy (kJ/mol) required to remove one (mole) ELECTRON(s) from one (mole) ISOLATED, GASEOUS atoms(s)/ion(s)
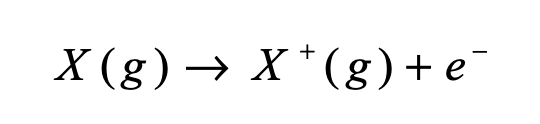
I.E (ionization energy) reflects how WHAT the electron is held by the WHAT in an atom
I.E (ionization energy) reflects how TIGHTLY the electron is held by the NUCLEUS in an atom
Sometimes I.E. is called “WHAT” = change in heat when removing an WHAT from an atom/ion
Sometimes I.E. is called “IONIZATION ENTHALPY” = change in heat when removing an ELECTRON from an atom/ion
I.E. is always WHAT (WHAT value)
I.E. is always ENDOTHERMIC (POSITIVE value)
First ionization energy (IE1) = energy to remove the most WHAT electron form a HWAT atom in WHAT state
First ionization energy (IE1) = energy to remove the most LOOSELY BOUND electron form a GASEOUS atom in GROUND state
First ionization energy
Going from left to right across the period, IE1, WHAT as the atomic number (WHAT nuclear charge) WHAT
First ionization energy
Going from left to right across the period, IE1, INCREASES as the atomic number (EFFECTIVE nuclear charge) INCREASES
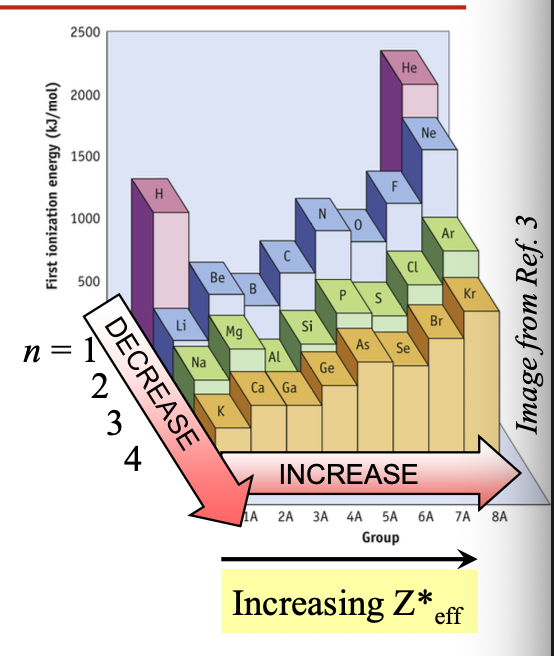
First ionization energy
Going from top to bottom in a group, IE1 WHAT as the period number (n) WHAT
First ionization energy
Going from top to bottom in a group, IE1 DECREASES as the period number (n) INCREASES
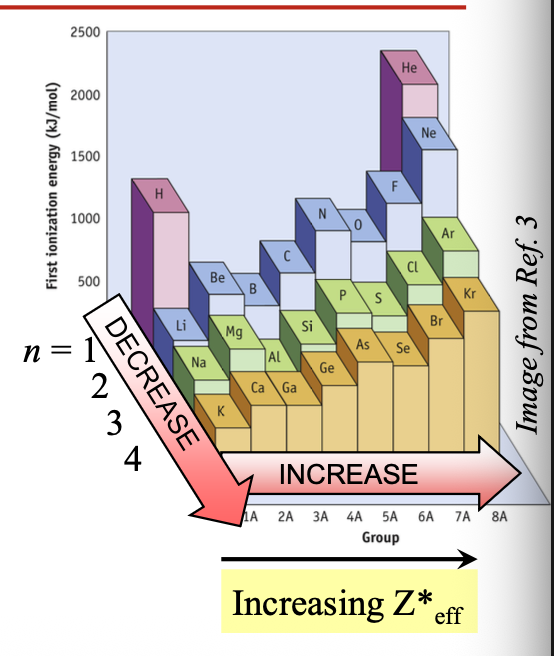
First ionization energy
Trends in IE are the WHAT of the trends in atomic size
First ionization energy
Trends in IE are the OPPOSITE of the trends in atomic size
First ionization energy
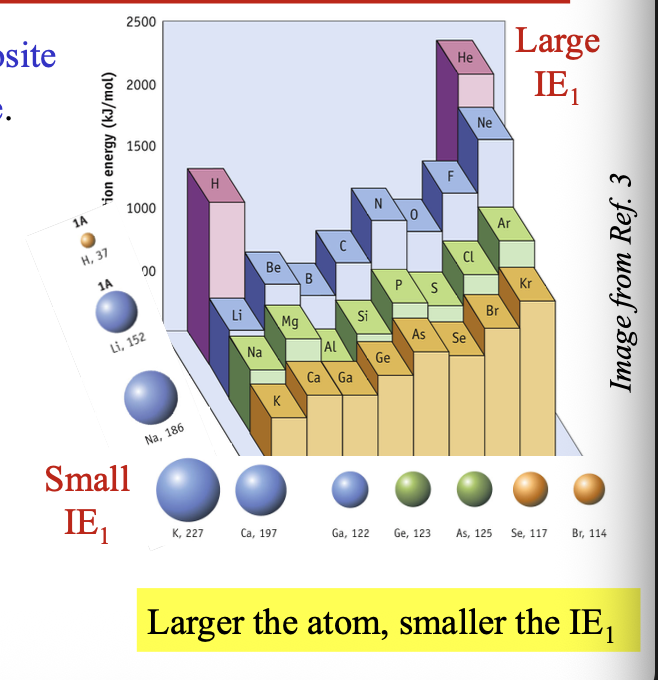
Non-metals have WHAT IE1, while metals have WHAT IE1
First ionization energy
Non-metals have LARGEST IE1, while metals have SMALLEST IE1
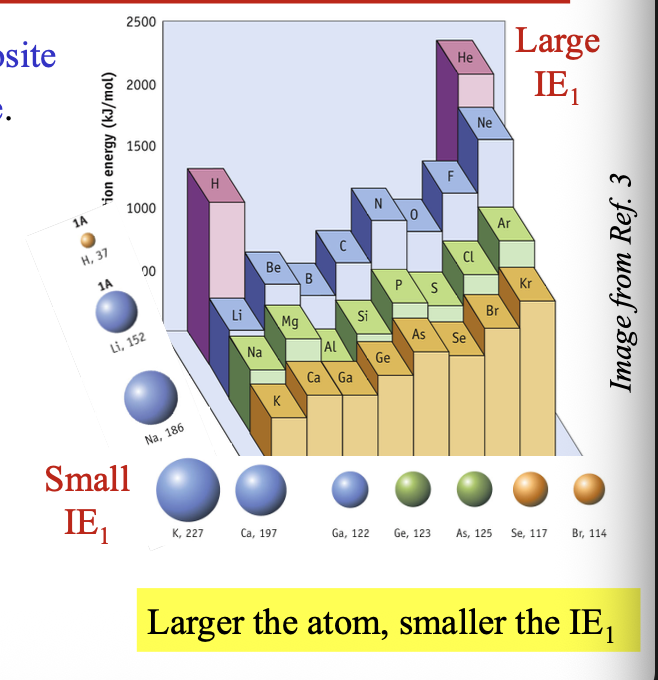
Larger the atom = WHAT the IE1
Larger the atom = SMALLER the IE1
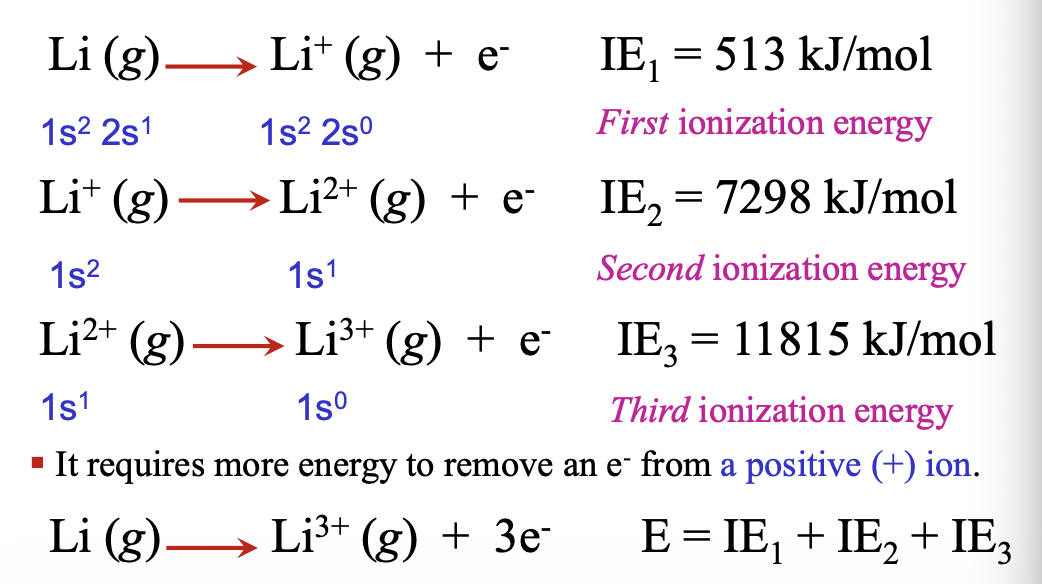
Successive ionizations are possible until no WHAT remains
Successive ionizations are possible until no ELECTRON remains
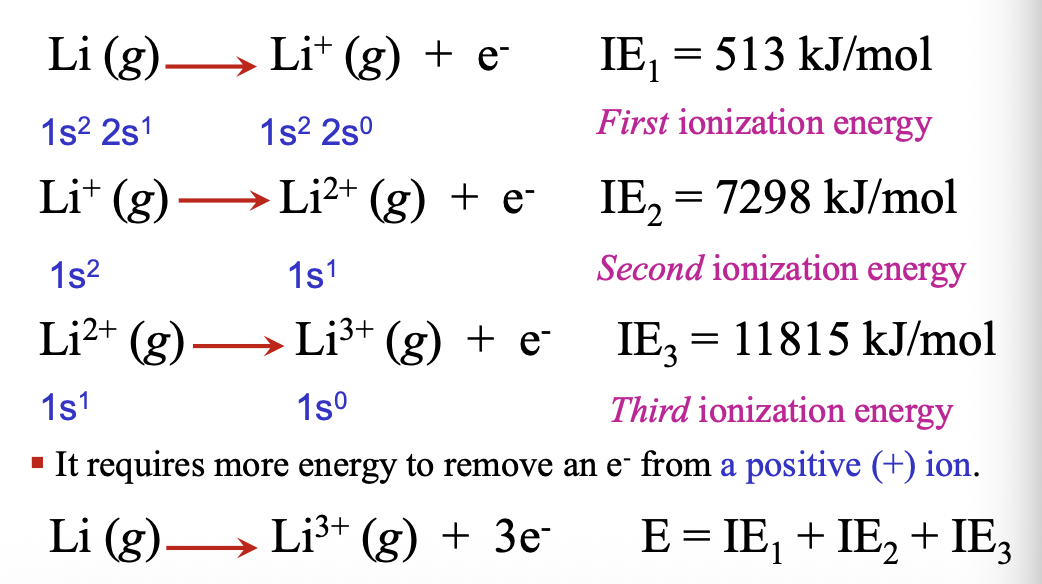
Successive ionization energies require more WHAT to remove an electron form a WHAt ion
Successive ionization energies require more ENERGY to remove an electron form a POSITIVE ion
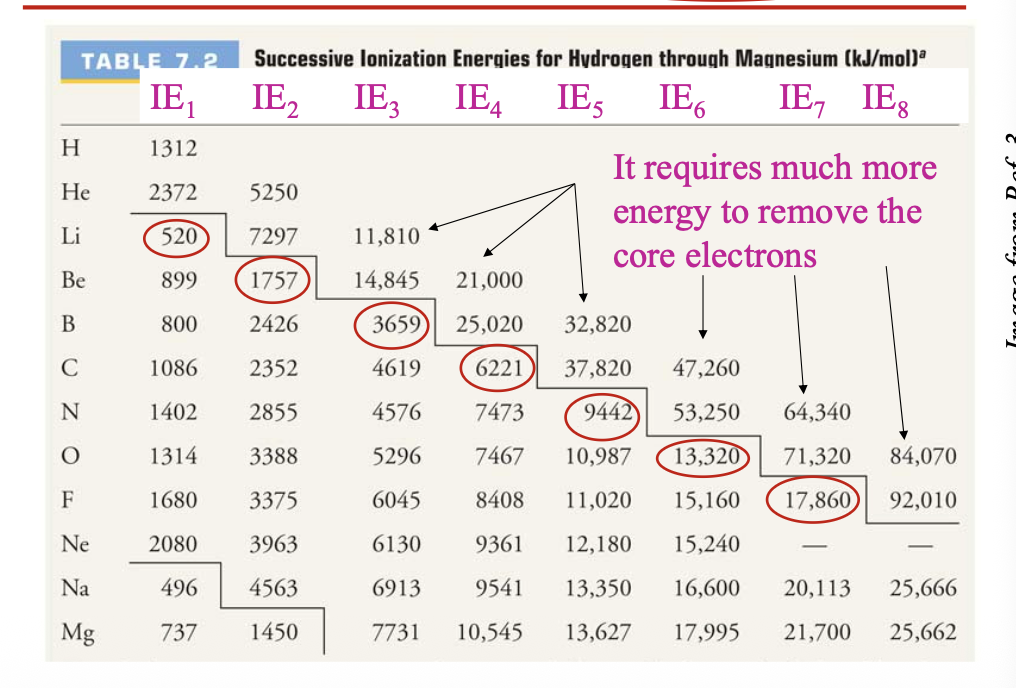
It requires much more energy to remove the WHAT electrons
It requires much more energy to remove the CORE electrons
Some atoms have an “WHAT” or “WHAT” for electrons
Some atoms have an “AFFINITY” or “LIKING” for electrons
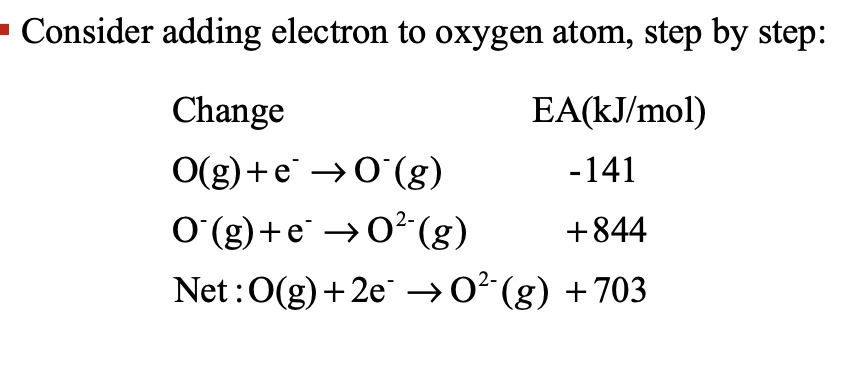
Electron affinity (E.A.) is the desire that a WHAT atom/ion has for adding an electron to its WHAT
Electron affinity (E.A.) is the desire that a GASEOUS atom/ion has for adding an electron to its VALENCE SHELL
Electron affinity is measured by the WHAT or WHAT (∆H in kJ/mol) that is absorbed or released when one (mole) electrons is added to one (mole) isolated, gaseous atom(s)/ion(s)
Electron affinity is measured by the HEAT or ENTHALPY (∆H in kJ/mol) that is absorbed or released when one (mole) electrons is added to one (mole) isolated, gaseous atom(s)/ion(s)
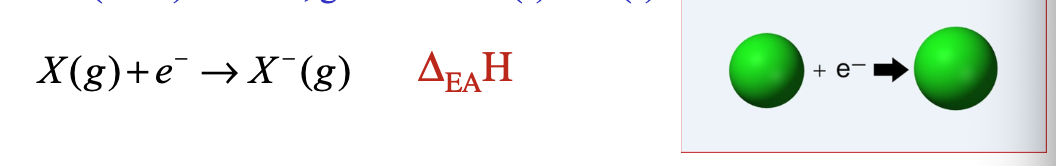
For almost all elements ∆(EA)H < 0 (WHAT, WHAT E.A.) but it can also be ∆(EA)H > 0 (WHAT, no WHAT)
For almost all elements ∆(EA)H < 0 (EXOTHERMIC, HIGH E.A.) but it can also be ∆(EA)H > 0 (ENDOTHERMIC, no DESIRE)
Going from left to right across a period, E.A. WHAT as the atomic number (effective nuclear charge) WHAT
Going from left to right across a period, E.A. INCREASES as the atomic number (effective nuclear charge) INCREASES
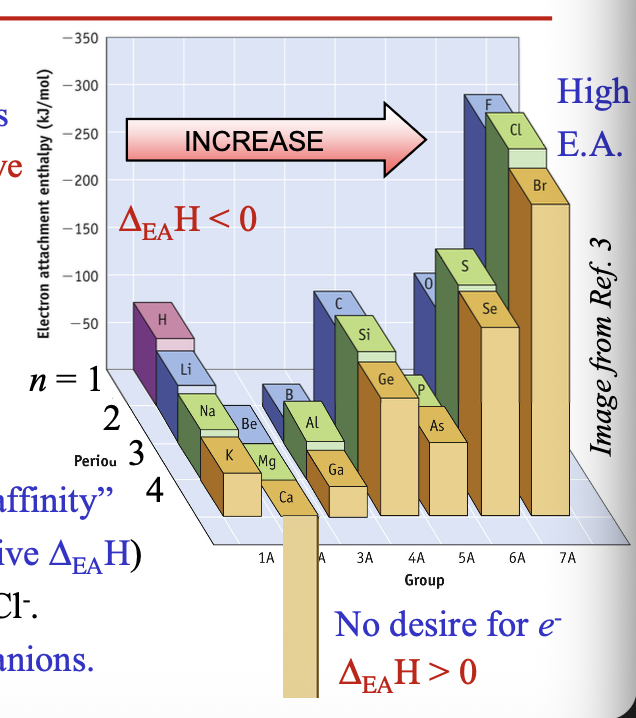
The more WHAT ∆(EA)H the WHAT the tendency of an atom to WHAT the electron
The more NEGATIVE ∆(EA)H the GREATER the tendency of an atom to ACCEPT the electron
WHAT have higher “affinity” for the electron ( more HWAT) forming, WHAT don’t like to form anions
NON-METALS have higher “affinity” for the electron ( more NEGATIVE ∆(EA)H) forming (eg, O-, S-, F- and Cl-), METALS don’t like to form anions
Addition of electrons to an WHAT with negative charge requires WHAT (∆(EA)H WHAT 0)
Addition of electrons to an ANION with negative charge requires ENERGY (∆(EA)H > 0)
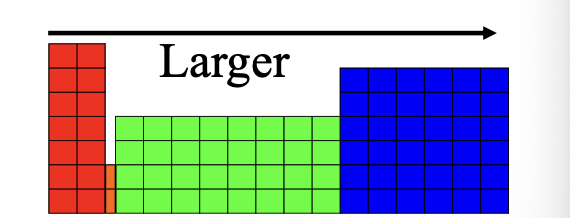
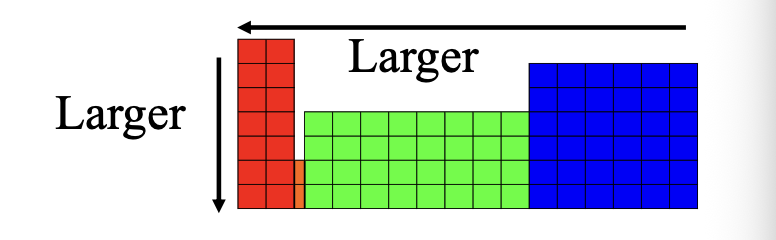
Atomic size on the periodic table for larger
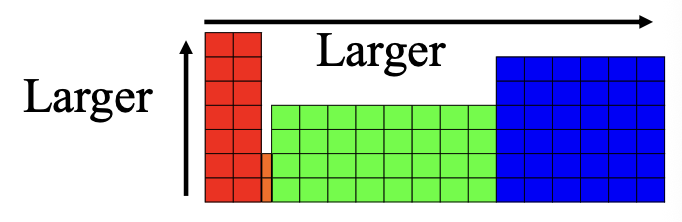
Ionization energy on the periodic table for larger
Electron affinity on the periodic table for larger