Chemistry Topic 1 Metals and the reactivity series
1/22
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
What is the reaction between metals and oxygen?
Metal + oxygen →metal oxide
What is the reaction between potassium and oxygen?
Burns with a lilac flame, forms a white solid
4K + O2 →2K2O
What is the reaction between sodium and oxygen?
Burns with a yellow flame, forms a white solid
4Na + O2 → 2Na2O
What is the reaction between calcium and oxygen?
Burns with a red flame, forms a red solid
2Ca + O2 → 2CaO
What is the reaction between magnesium and oxygen?
Burns with a bright white light, forms a white solid
2Mg + O2 → 2MgO
What is the reaction between aluminium and oxygen?
Burns only when a fine powder, forms white solid
4Al + 3O2 → 2Al2O3
What is the reaction between zinc and oxygen?
Burns in air forming a yellow solid which changes to white on cooling
2Zn + O2 → 2ZnO
What is the reaction between iron and oxygen?
Iron fillings burn with orange sparks, forms black solid
3Fe + 2O2 → Fe3O4
What is the reaction between Copper and oxygen?
Doesn’t burn but becomes covered in black layer
2Cu + O2 → 2CuO
What is the reaction between metals and water?
metal + water → metal hydroxide + hydrogen
What is the reaction between lithium and water?
Floats on surface
Moves about surface
Fizzes, gas given off
Eventually disappears
Heat given out
Colourless solution formed
2Li + 2H2O → 2LiOH + H2
What is the reaction between sodium and water?
Floats on surface
Melts into silvery ball
Moves about surface
Burns with yellow flame
Fizzes, gas given off
Eventually disappears
Heat given off
Colourless solution forms
2Na + 2H2O → 2NaOH + H2
What is the reaction between potassium and water?
Floats on surface
Moves about surface
Burns with lilac flame
Fizzes, gas given off
Eventually disappears
Heat given out
Colourless solution formed
2K + 2H2O → 2KOH + H2
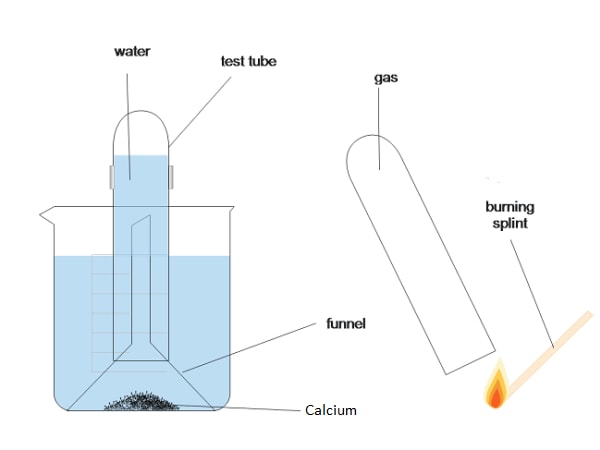
What is the reaction between calcium and water?
Sinks to bottom of the beaker, then rises
Effervescence
Heat released
Gets smaller then disappears
Colourless solution forms with white precipitate
Ca + 2H2O → Ca(OH)2 + H2
What is the reaction between magnesium and water?
A few bubbles of gas produced over time
Mg + 2H2O → Mg(OH)2 + H2
What is the reaction between metals and steam?
metal + steam → metal oxide + hydrogen
What is the reaction between magnesium and steam?
Bright white light
White solid forms
Heat given out
Mg + H2O → MgO + H2
What is the reaction between Aluminium and steam?
Forms white solid
Heat given out
2Al + 3H2O → Al2O3 + 3H2
What is the reaction between zinc and steam?
Glows to form yellow powder
Forms a white powder once cooled
Heat given out
Zn + H2O → ZnO + H2
What is the reaction between iron and steam?
Powdered iron glows to form a black solid
3Fe + 4H2O → Fe3O4 + 4H2
What is the reactivity series?
Perfectly Safe Chemists Must Always Zoom In Carefully
Potassium
Sodium
Calcium
Magnesium
Aluminium
Zinc
Iron
Copper
What is a displacement reaction?
When a more reactive metal takes the place of a less reactive metal in a compound
What is the reaction between magnesium and copper sulfate?
Blue solution fades
Red-brown solid forms
Heat is released
Colourless solution forms
Mg + CuSO4 → MgSO4 + Cu