S2 IB Chemistry HL
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
Anion
Negatively charged ion
Cation
Positively charged ion
Ionic bond
the electrostatic attraction between oppositely charged ion
Lattice
3D structure of ions
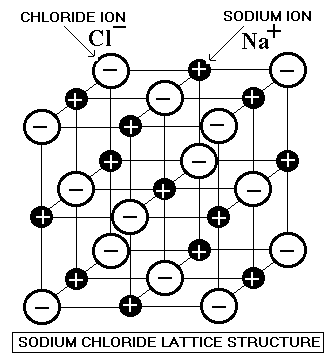
Lattice enthalpy
The measure of electrostation attraction
(Directly proportional)
Larger radius
More occupied energy level therefore weaker electrostatic attraction between nucleus therefore weaker bond (Indirectly proportional)
Greater charge
Greater attraction between ions therefore stronger bond (Direc)tly proportional)
Physical properties of Ions
Low volatility (ability to vapourise)
High Melting point and Boiling point
Most is soluble in polar solvent
Good conductor when liquid, Poor conductor when solid (of electricity)
Brittle → layers are easily shifted and fractured