Strong and weak acids
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
What is meant by a strong acid?
Strong acids fully dissociate into ions when they dissolve in water. There are no molecules of the original acid left, and the change is not reversible
Give some examples of strong acids:
Hydrochloric, nitric and sulfuric acids
What is meant by a weak acid?
Weak acids do not fully dissociate into ions when they dissolve in water. Only some of the original acid molecules dissociate into ions, and the change is reversible
Give some examples of weak acids:
Ethanoic, citric and carbonic acids
What is the chemical formula for hydrochloric acid?
HCl

What is the chemical formula for sulfuric acid?
H2SO4
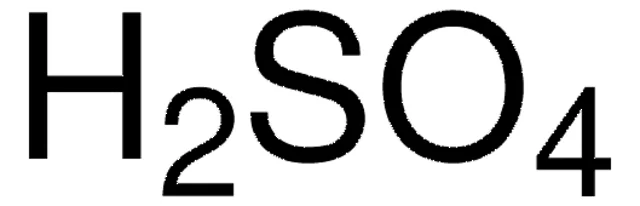
What is the chemical formula for nitric acid?
HNO3
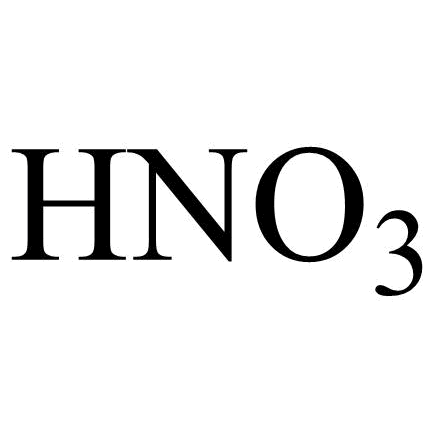
What is the chemical formula for citric acid?
C6H8O7

What is the formula for carbonic acid?
H2CO3
What happens to pH if the H⁺ concentration increases by a factor of 10?
The pH decreases by 1
What happens to pH if the H⁺ concentration decreases by a factor of 10?
The pH increases by 1
Define what is meant by a concentrated solution?
Concentrated solutions have a large number of solute particles dissolved per dm cubed of the solution
Define what is meant by a dilute solution:
Dilute solutions have a low number of solute particles dissolved per dm cubed of the solution
How does a high concentration of H+ ions affect pH?
A high conc. of H+ ions means a low pH
What does a lower concentration of H+ ions mean?
A lower concentration of H+ ions means a higher (more neutral) pH
Explain how diluting an acidic solution changes the pH in terms of H+ ions
Diluting an acidic solution reduces/lowers the concentration of the H+ ions