MCAT Biochemistry - Carbohydrate Metabolism I: Glycolysis, Glycogen, Gluconeogenesis, and the Pentose Phosphate Pathway
1/76
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
77 Terms
Glucose entry into cells
driven by concentration and is independent of sodium
Normal glucose concentration in peripheral blood
5.6 mM (normal range: 4–6 mM)
glucose transporters
4, GLUT 1 through GLUT 4
GLUT 2
low-affinity transporter in hepatocytes and pancreatic cells; captures the excess glucose primarily for storage; pick up glucose in proportion to its concentration in the blood (first-order kinetics); along with the glycolytic enzyme glucokinase, serves as the glucose sensor for insulin release
Km = ~15 mM
GLUT 4
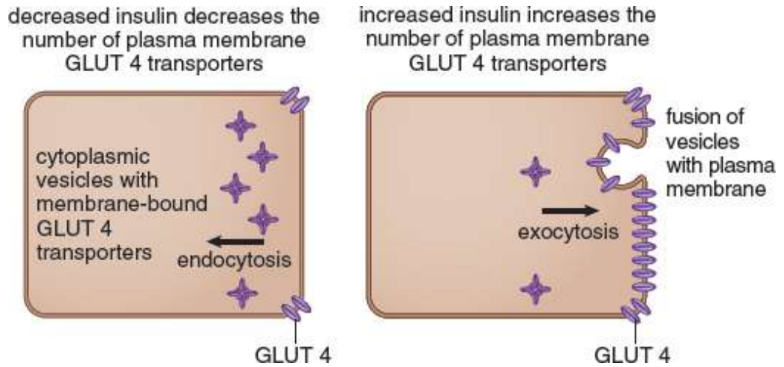
in adipose tissue and muscle; responds to the glucose concentration in peripheral blood; constant rate of glucose influx because they will be saturated if blood glucose is even slightly higher (zero-order kinetics); insulin stimulates the movement of additional transporters to the membrane by exocytosis
Km= 5 mM (normal glucose levels in blood)
Glycolysis
cytoplasmic pathway that converts glucose into two pyruvate molecules, releasing a modest amount of energy captured in two substrate-level phosphorylations and one oxidation reaction; may occur anaerobically, although some of the available energy is lost
all cells must be able to do it; RBC lack mitochondria and depend on this for energy, cancer cells use this more often than healthy cells
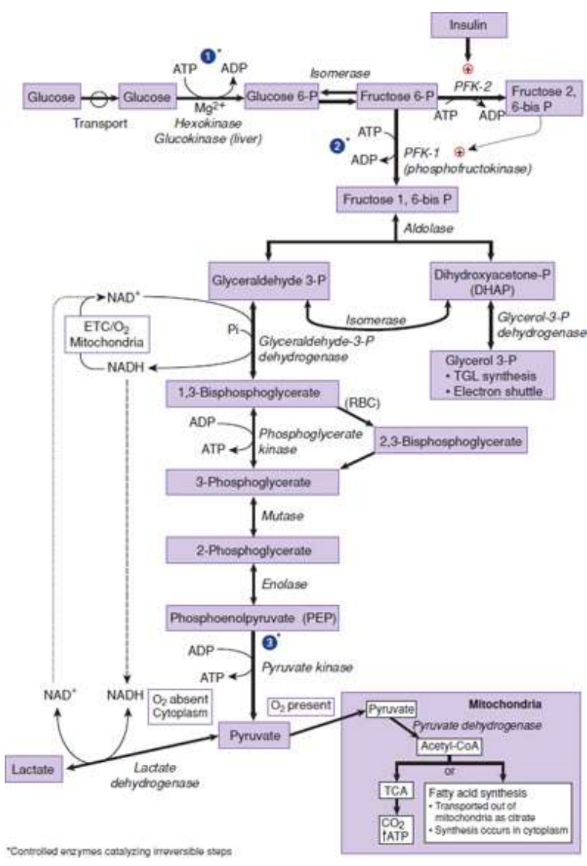
Hexokinase
phosphorylates glucose to prevent leaving via the transporter
widely distributed in tissues
Low Km
inhibited by its product, glucose 6-phosphate
irreversible
Glucokinase
phosphorylates glucose to prevent leaving via the transporter
found only in liver cells and pancreatic β-islet cells
High Km
Induced by insulin in hepatocytes
irreversible
rate-limiting enzymes for Carbohydrate Metabolism Pathways
Glycolysis: phosphofructokinase-1
Fermentation: lactate dehydrogenase
Glycogenesis: glycogen synthase
Glycogenolysis: glycogen phosphorylase
Gluconeogenesis: fructose-1,6-bisphosphatase
Pentose Phosphate Pathway: glucose-6-phosphate dehydrogenasen
Phosphofructokinase-1 (PFK-1)
rate-limiting enzyme and main control point in glycolysis: irreversible
fructose 6-phosphate is phosphorylated to fructose 1,6-bisphosphate using ATP
inhibited by ATP (high energy) and citrate (intermediate of citric acid cycle), and activated by AMP (low energy) and fructose 2,6-bisphosphate (F2,6-BP) (allows these cells to override the inhibition caused by ATP)
Insulin stimulates and glucagon inhibits in hepatocytes
Phosphofructokinase-2 (PFK-2)
converts a tiny amount of fructose 6-phosphate to fructose 2,6-bisphosphate (F2,6-BP)
Insulin stimulates and glucagon inhibits
mostly found in liver
Glyceraldehyde-3-phosphate dehydrogenase
catalyzes an oxidation and addition of inorganic phosphate (Pi) to its substrate, glyceraldehyde 3- phosphate → production of a high-energy intermediate 1,3-bisphosphoglycerate and the reduction of NAD+ to NADH
3-Phosphoglycerate kinase
transfers the high-energy phosphate from 1,3-bisphosphoglycerate to ADP, forming ATP and 3-phosphoglycerate
substrate-level phosphorylation
ADP is directly phosphorylated to ATP using a high-energy intermediate; not dependent on oxygen; only means of ATP generation in an anaerobic tissue
Pyruvate Kinase
last enzyme in aerobic glycolysis; catalyzes a substrate-level phosphorylation of ADP using the high-energy substrate phosphoenolpyruvate (PEP); irreversible
activated by fructose 1,6-bisphosphate from PFK-1
feed-forward activation
the product of an earlier reaction stimulates, or prepares, a later reaction
fermentation
anaerobic method of replenishing NAD+
lactate dehydrogenase
oxidizes NADH to NAD+, replenishing the oxidized coenzyme for glyceraldehyde-3-phosphate dehydrogenase
reduces pyruvate to lactate, both three-carbon molecules
used when oxygenation is poor (during strenuous exercise in skeletal muscle, a heart attack, or a stroke)
yeast fermentation
conversion of pyruvate (three carbons) to ethanol (two carbons) and carbon dioxide (one carbon); replenishing NAD+
Dihydroxyacetone phosphate (DHAP)
used in hepatic and adipose tissue for triacylglycerol synthesis; formed from fructose 1,6-bisphosphate; isomerized to glycerol 3-phosphate, which can then be converted to glycerol
1,3-Bisphosphoglycerate (1,3-BPG)
high-energy intermediates used to generate ATP by substrate-level phosphorylation using 3-Phosphoglycerate kinase; only ATP gained in anaerobic respiration
phosphoenolpyruvate (PEP)
high-energy intermediates used to generate ATP by substrate-level phosphorylation using Pyruvate Kinase; only ATP gained in anaerobic respiration
Adaptation to high altitudes (low pO2)
Increased respiration
Increased oxygen affinity for hemoglobin (initial)
Increased rate of glycolysis
Increased [2,3-BPG] in RBC (over a 12–24 hour period)
Normalized oxygen affinity for hemoglobin restored by the increased level of 2,3-BPG
Increased hemoglobin (over days to weeks)
bisphosphoglycerate mutase
in RBC, produces 2,3-bisphosphoglycerate from 1,3-BPG; moves phosphate group from 1-position to 2-position
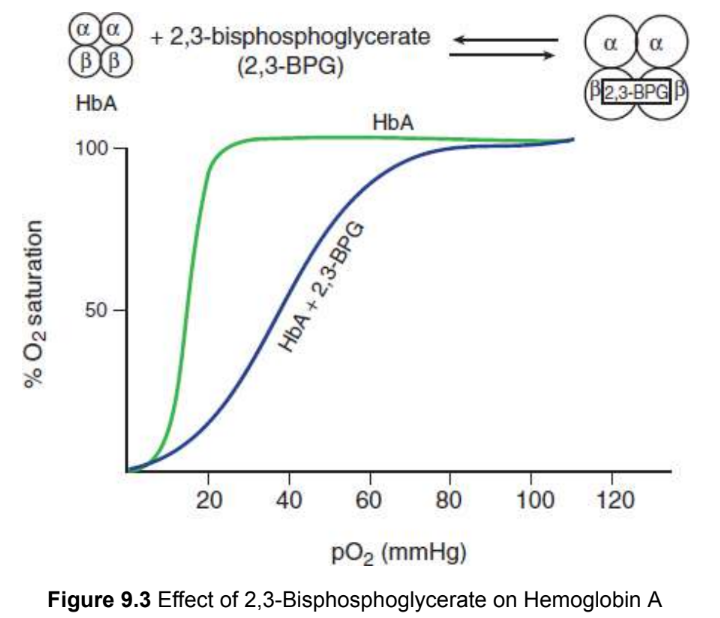
2,3-bisphosphoglycerate (2,3-BPG)
binds allosterically to the β-chains of hemoglobin A (HbA) and decreases its affinity for oxygen; rightward shift in the curve is sufficient to allow unloading of oxygen in tissues, but still allows 100 percent saturation in the lungs; does not bind well to fetal hemoglobin `(HbF)
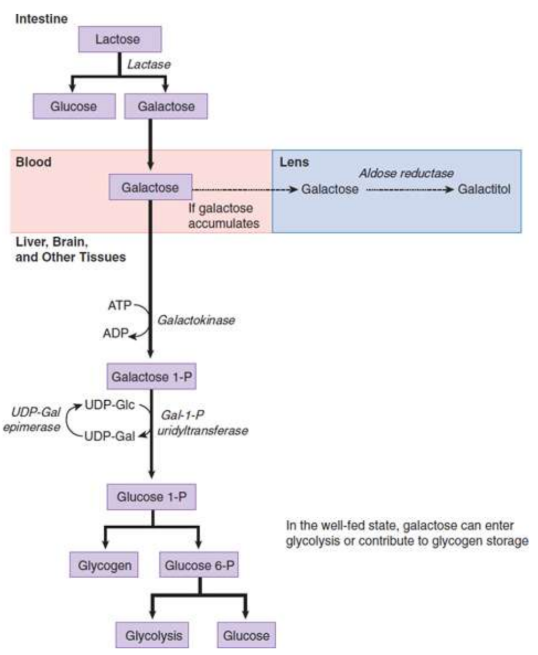
lactose/galactose metabolism
sucrose/fructose metabolism
Note:
Galactokinase
Galactose-1-phosphate uridyltransferase
galactose
derived from lactose by lactase; reaches the liver through the hepatic portal vein
Lactose
sugar found in milk; hydrolyzed to galactose and glucose by lactase
lactase
brush-border enzyme of the duodenum; hydrolyses lactose to galactose and glucose
galactokinase
phosphorylates galactose to galactose 1-phosphate; trapping it in hepatocytes
galactose-1-phosphate uridyltransferase
onverts galactose 1-phosphate to glucose 1-phosphate with an epimerase
Epimerases
enzymes that catalyze the conversion of one sugar epimer to another
epimers
diastereomers that differ at exactly one chiral carbon
galactosemia.
Genetic deficiencies of galactokinase or galactose-1-phosphate uridyltransferase; lead to cataracts
Cataracts
conversion of excess galactose in the blood to galactitol in the lens of the eye by aldose reductase; ; Accumulation of galactitol in the lens causes osmotic damage
polyol
a carbon chain with many alcohol groups
Primary lactose intolerance
hereditary deficiency of lactase; bacterial fermentation of lactose, which produces a mixture of CH4, H2, and small organic acids; result in the movement of water into the intestinal lumen
symptoms: include vomiting, bloating, explosive and watery diarrhea, cramps, and dehydration
Secondary lactose intolerance
precipitated at any age by gastrointestinal disturbances that cause damage to the intestinal lining, where lactase is found; bacterial fermentation of lactose, which produces a mixture of CH4, H2, and small organic acids; result in the movement of water into the intestinal lumen
symptoms: include vomiting, bloating, explosive and watery diarrhea, cramps, and dehydration
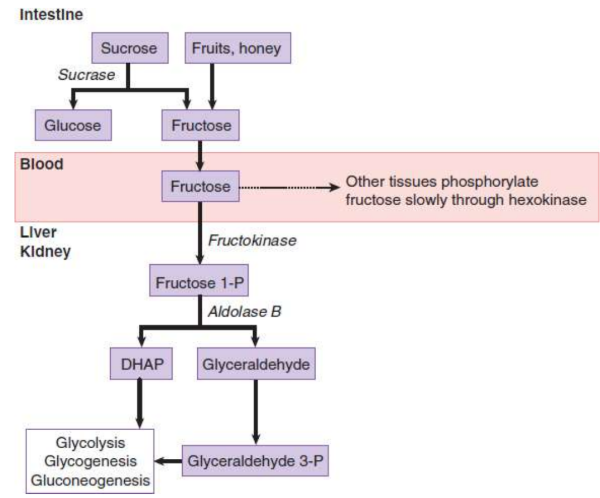
Fructose
found in honey and fruit and part of sucrose; absorbed into the hepatic portal vein; also metabolized in renal proximal tubules
sucrose
common table sugar; hydrolysed by glucose and fructose by sucrase
sucrase
duodenal brush-border enzyme; hydrolyses sucrose into glucose and fructose
fructokinase
phosphorylates fructose to fructose 1-phosphate in hepatocytes
aldolase B
cleaves fructose 1-phosphate into glyceraldehyde and DHAP; products downstream from PFK in gkycolysis
dihydroxyacetone phosphate (DHAP)
products of fructose metabolism
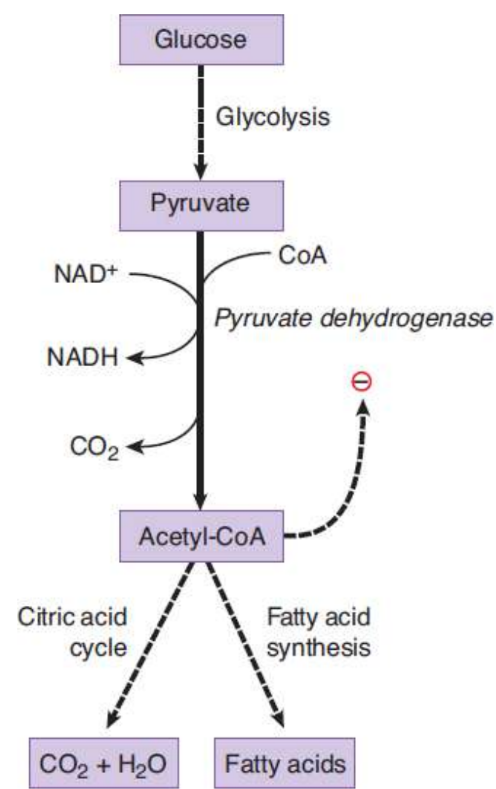
acetyl-CoA
product of pyruvate; necessary for citric acid cycle
pyruvate dehydrogenase complex (PDH)
converts pyruvate to Acetyl CoA; irreversible; requires multiple cofactors and coenzymes, including thiamine pyrophosphate, lipoic acid, CoA, FAD, and NAD+
activated by insulin in liver; not responsive to hormones in nervous system
Glycogen
a branched polymer of glucose used for storage; primarily in liver (source of glucose that is mobilized between meals to prevent low blood sugar) and skeletal muscle (stored as an energy reserve for muscle contraction); stored in the cytoplasm as granules: composed entirely of linear chains have the highest density of glucose near the core, while branched glucose density is highest at the periphery of the granule, allowing more rapid release of glucose on demand

starch
long α-linked chains of glucose; plant storage of excess sugar

Glycogenesis
synthesis of glycogen granules; begins with a core protein called glycogenin
begins with glucose 6-phosphate converted to glucose 1-phosphate
glucose 1-phosphate is then activated by coupling to a molecule of uridine diphosphate (UDP)
integration into the glycogen chain by glycogen synthase when glucose 1-phosphate interacts with uridine triphosphate (UTP)
forming UDP-glucose and a pyrophosphate (PPi)
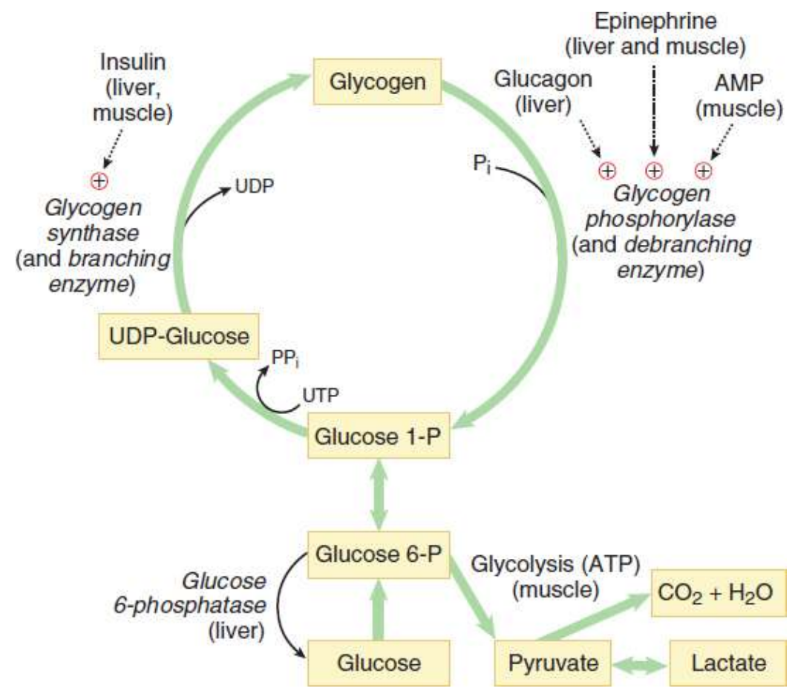
Glycogen synthase
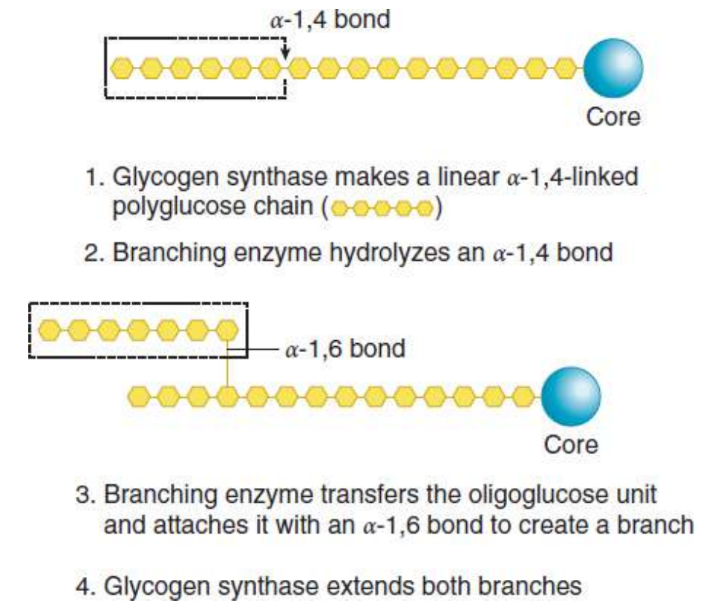
rate-limiting enzyme of glycogen synthesis; forms the α-1,4 glycosidic bond found in the linear glucose chains of the granule
stimulated by glucose 6-phosphate and insulin
inhibited by epinephrine and glucagon through a protein kinase phosphorylation
Branching enzyme
introduces α-1,6-linked branches into the granule as it grows; hydrolyzes one of the α-1,4 bonds to release a block of oligoglucose, which is then moved and added in a slightly different location where it forms an α-1,6 bond to create a branch
glycogenolysis
process of breaking down glycogen
glycogen phosphorylase
rate-limiting enzyme of glycogenolysis; breaks only α-1,4 glycosidic bonds, releasing glucose 1- phosphate from the periphery of the granule using an inorganic phosphate; forms glucose 1-phosphate then converted to glucose 6-phosphate by mutase
activated by glucagon in the liver, activated by AMP and epinephrine in skeletal muscle
inhibited by ATP
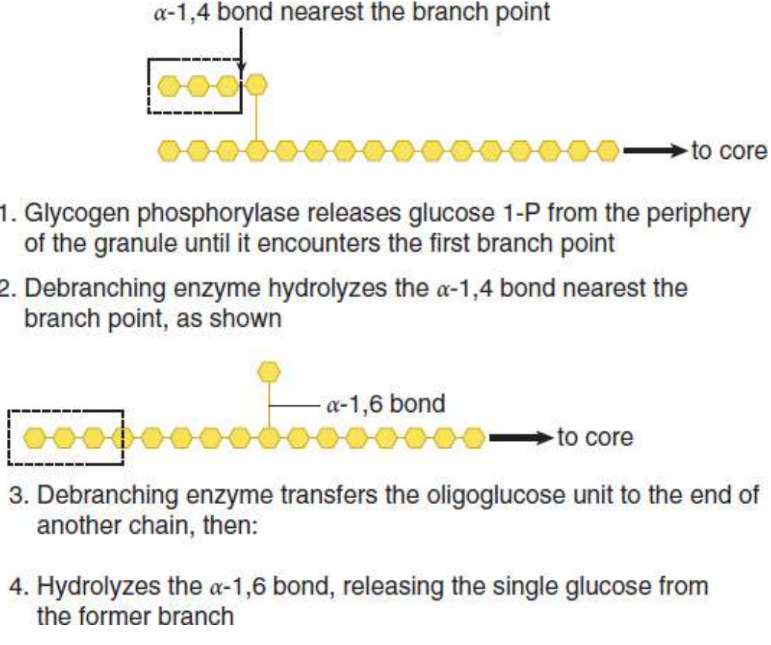
Debranching enzyme
two-enzyme complex that deconstructs the branches in glycogen that have been exposed by glycogen phosphorylase; Breaks an α-1,4 bond adjacent to the branch point and moves the small oligoglucose chain that is released to the exposed end of the other chain; Forms a new α-1,4 bond; Hydrolyzes the α-1,6 bond, releasing the single residue at the branch point as free glucos
von Gierke’s disease
defect in glucose-6-phosphatase; periods of extremely low blood sugar between meals; buildup of glucose 6-phosphate enlarges and damages liver over time
Isoforms
slightly different versions of the same protein
glycogen storage diseases
metabolic genetic deficiencies characterized by accumulation or lack of glycogen in one or more tissues
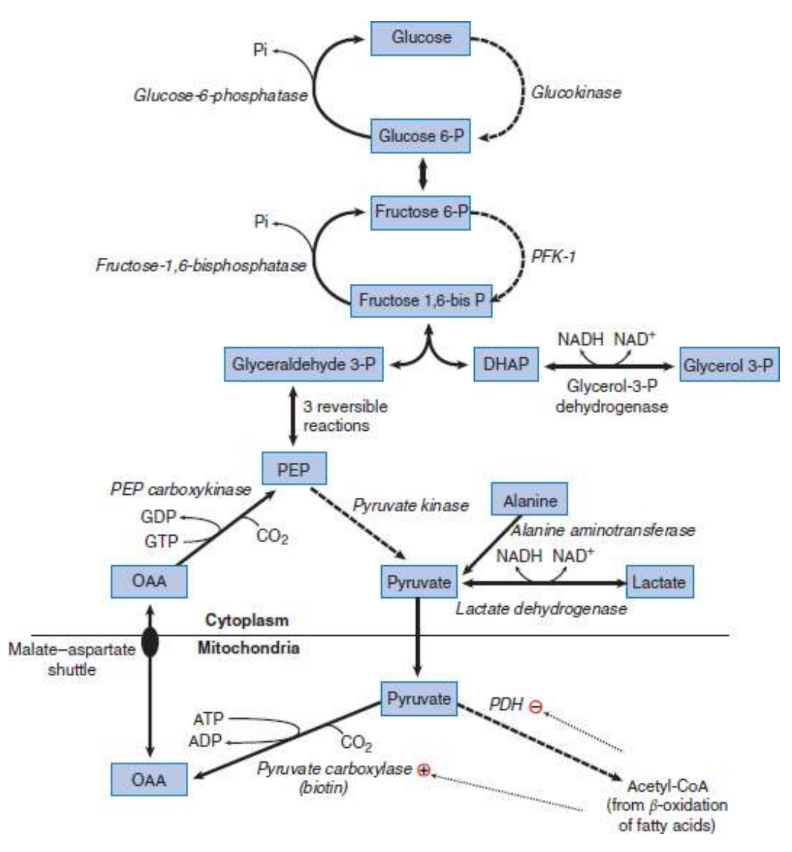
gluconeogenesis
metabolic pathway that results in the biosynthesis of glucose from certain non-carbohydrate carbon substrates; liver and kidney; requires expenditure of ATP that is provided by β-oxidation of fatty acids
promoted by glucagon and epinephrine
inhibited by insulin
fasting
glycogen reserves drop dramatically in the first 12 hours
gluconeogenesis increases
After 24 hours, it represents the sole source of glucose
Glucogenic amino acids
can be converted by individual pathways to citric acid cycle intermediates, then to malate, following the same path from there to glucose
all except leucine and lysine, esp. alanine
ketogenic amino acids
can be converted into ketone bodies, which can be used as an alternative fuel
Important substrates for gluconeogenesis
Glycerol 3-phosphate (from stored fats, or triacylglycerols, in adipose tissue)
Lactate (from anaerobic glycolysis)
Glucogenic amino acids (from muscle proteins)
lactate dehydrogenase
Lactate is converted to pyruvate
alanine aminotransferase
Alanine is converted to pyruvate
glycerol-3-phosphate dehydrogenase
Glycerol 3-phosphate is converted to dihydroxyacetone phosphate (DHAP)
Pyruvate carboxylase
mitochondrial enzyme that is activated by acetyl-CoA (from β-oxidation); product, oxaloacetate (OAA), is a citric acid cycle intermediate and cannot leave the mitochondrion; reduced to malate, which can leave the mitochondrion via the malate–aspartate shuttle
Phosphoenolpyruvate carboxykinase (PEPCK)
converts OAA to phosphoenolpyruvate (PEP); requires GTP; PEP continues in the pathway to fructose 1,6-bisphosphate
cytoplasm
induced by glucagon and cortisol
Fructose-1,6-bisphosphatase
key control point of gluconeogenesis; represents the rate-limiting step of the process; reverses the action of phosphofructokinase-1 by removing phosphate from fructose 1,6-bisphosphate to produce fructose 6-phosphate
activated by ATP
inhibited by AMP and fructose 2,6-bisphosphate
Glucose-6-phosphatase
Glucose 6-phosphate is transported into the ER, and free glucose is transported back into the cytoplasm where it can diffuse out of the cell using GLUT transporters
lumen of the endoplasmic reticulum in liver cells
pentose phosphate pathway (PPP) / hexose monophosphate (HMP) shunt
production of NADPH and serving as a source of ribose 5-phosphate for nucleotide synthesis
glucose-6-phosphate dehydrogenase; begins with glucose 6-phosphate, ends with ribulose 5-phosphate; irreversible → NADPH
transketolase and transaldolase; beginning with ribulose 5-phosphate, reversible reactions that produce an equilibrated pool of sugars for biosynthesis, including ribose 5-phosphate; can feed back into glycolysis
cytoplasm of all cells
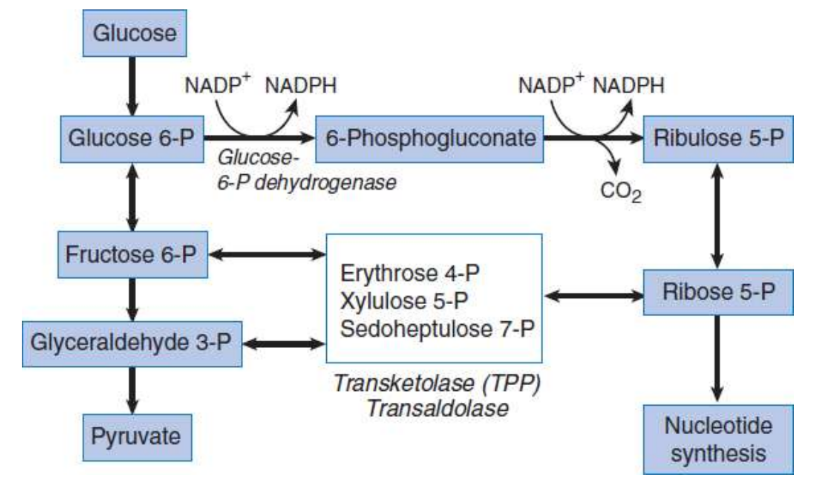
glucose-6-phosphate dehydrogenase (G6PD)
rate-limiting enzyme of pentose phosphate pathway; first step
induced by insulin
activated by one of its reactants, NADP+
inhibited by its product, NADPH
G6PD deficiency / favism
X-linked disorder; most common inherited enzyme defect
susceptible to oxidative stress, certain oxidizing compounds (antibiotics, antimalarial medications, fava beans) or infections can lead to high concentrations of reactive oxygen species
some malaria resistance
ribose 5-phosphate
backbone of nucleic acids; isomerised from ribulose 5-phosphate in PPP
NADPH
electron donor in a number of biochemical reactions; potent reducing agent
Biosynthesis, mainly of fatty acids and cholesterol
Assisting in cellular bleach production in certain white blood cells, thereby contributing to bactericidal activity
Maintenance of a supply of reduced glutathione to protect against reactive oxygen species (acting as the body’s natural antioxidant)
Hydrogen peroxide, H2O2
produced as a byproduct in aerobic metabolism, and can break apart to form hydroxide radicals, OH•, that can attack lipids, including those in the phospholipids of the membrane
Glutathione
reducing agent that can help reverse radical formation before damage is done to the cell