Chemistry atom evolution and dalton's theory
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Dalton (1st), atoms=indivisible, identical by elements, compounds made by atoms
What is this model? (Name, Theory, Experiment)

Thomson (2nd), Atoms are composed of electrons that are scattered throughout a positively charged “soup”, Cathode Ray Tube
What is this model? (Name, Theory, Experiment)
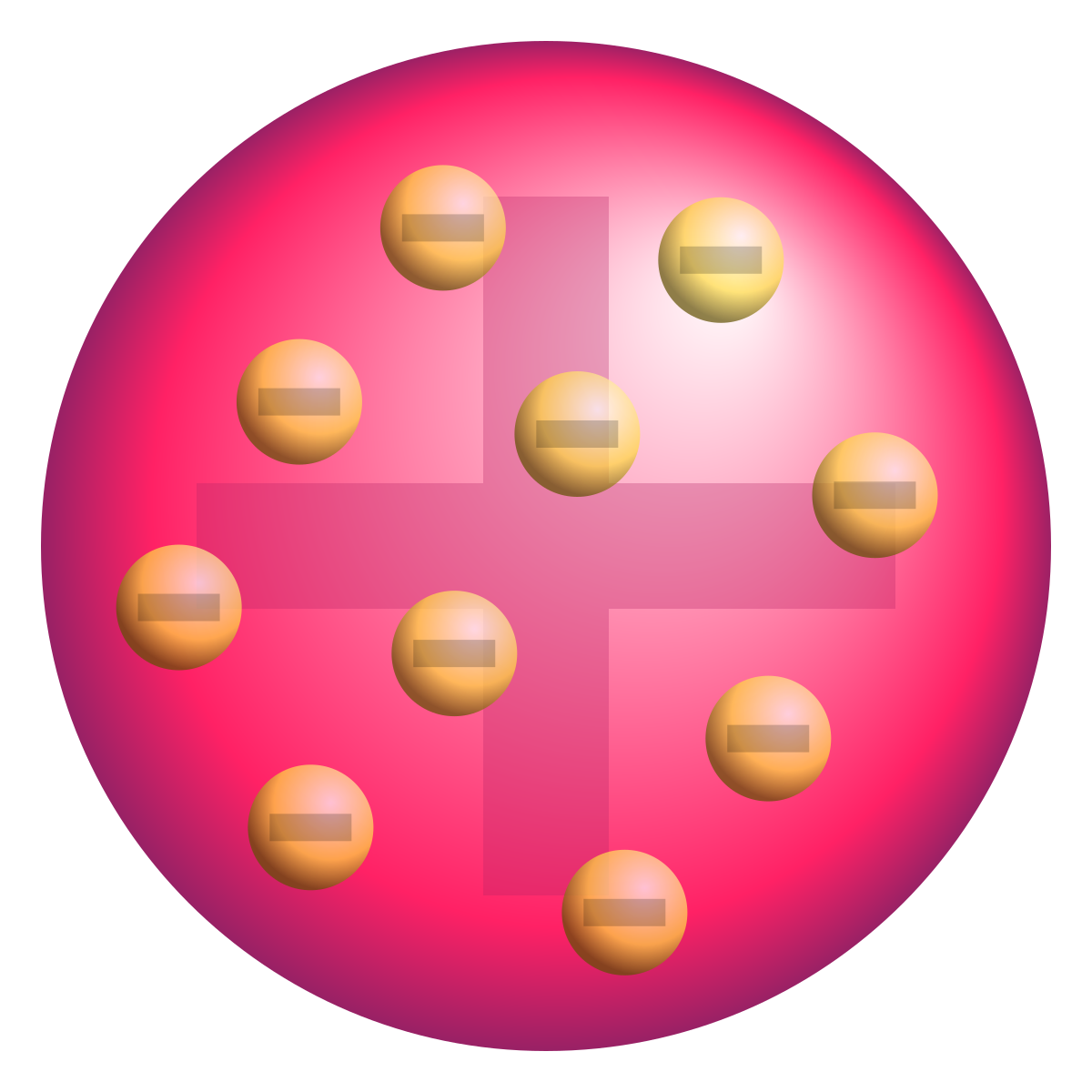
Rutherford (3rd), Atoms are mostly empty space and positive charge concentrated in the center (nucleus), Gold Foil Experiment
What is this model? (Name, Theory, Experiment)

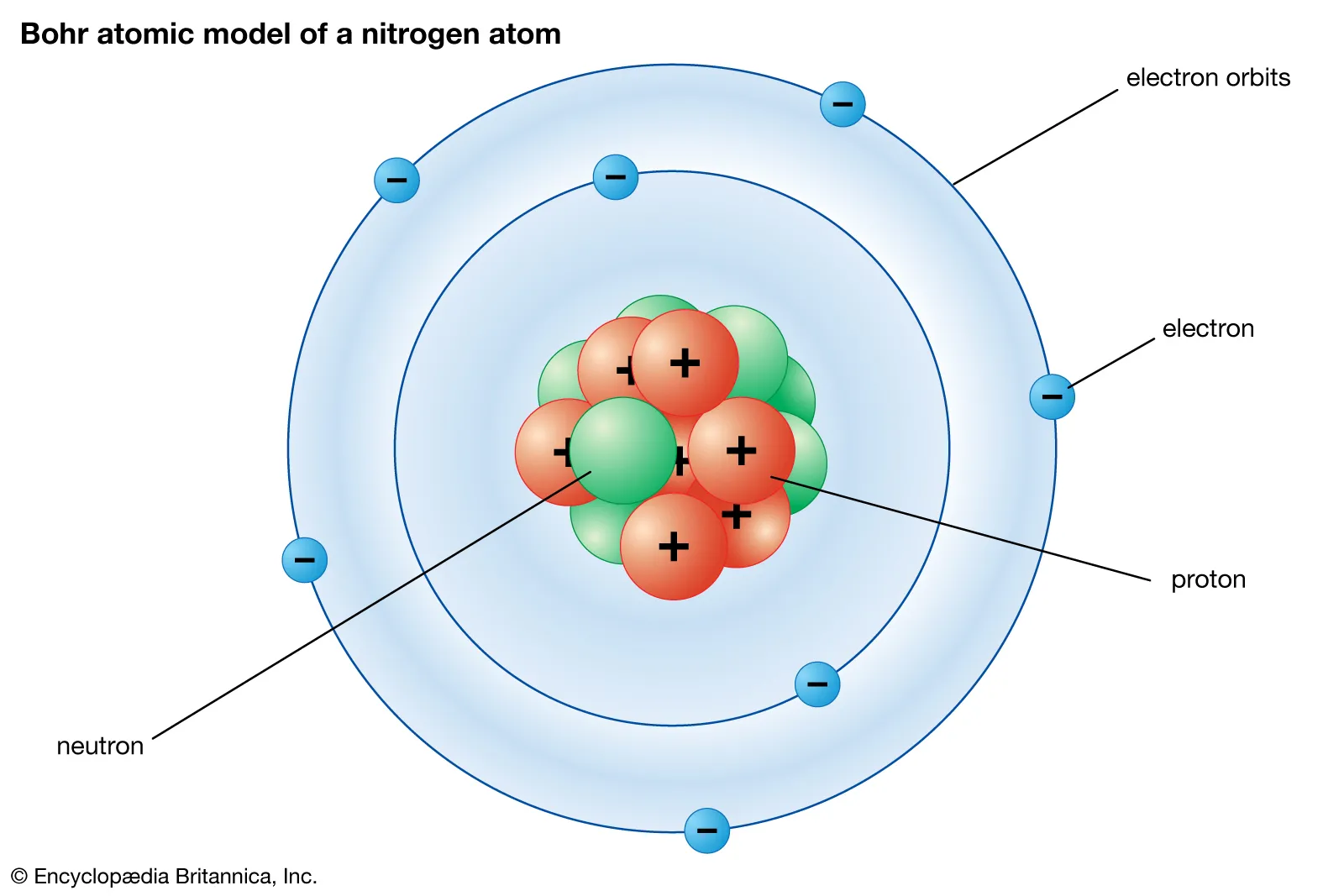
Bohr (4th), Elements move in fixed orbits that differ in size and energy, hydrogen spectroscopy
What is this model? (Name, Theory, Experiment)
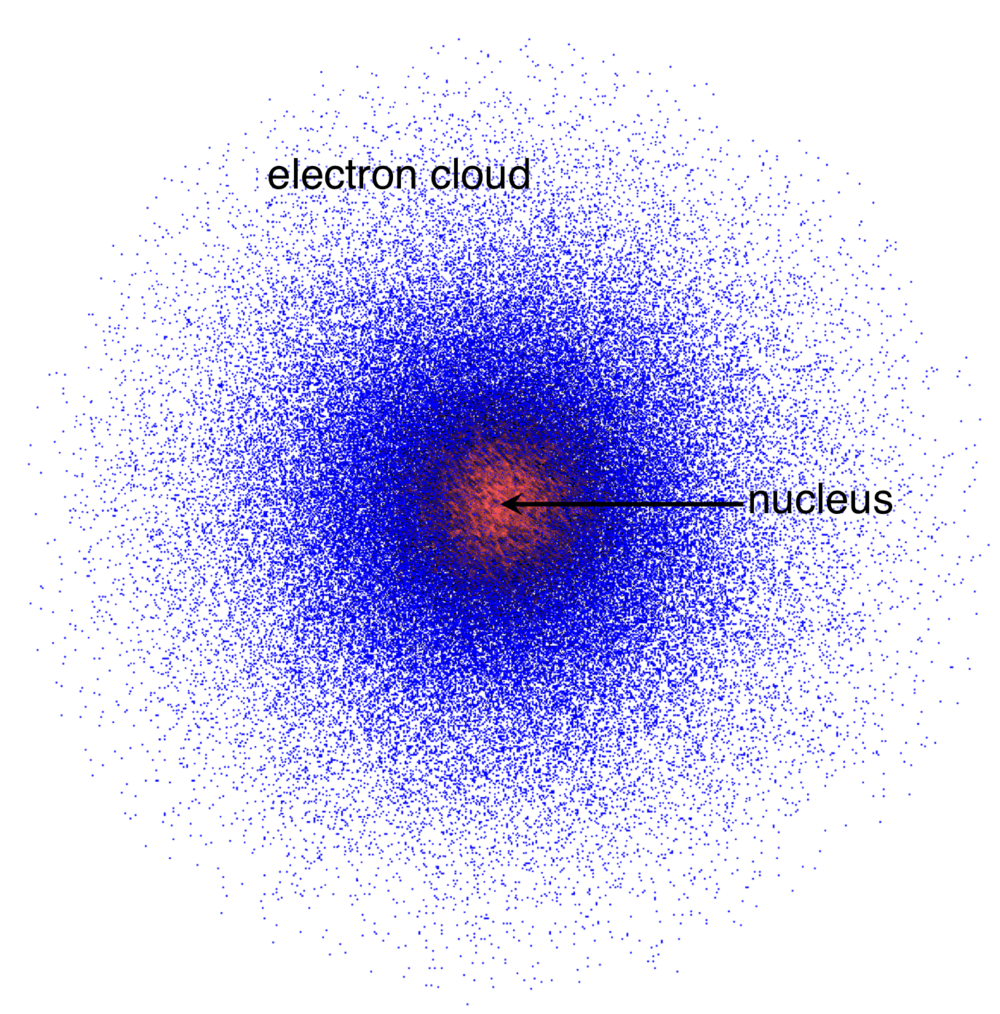
Schrödinger, “Clouds of probability”- electrons move in a wave and it is impossible to know the exact location
What is this model? (Name, Theory, Experiment)
Dalton’s Atomic Theory
1) Atoms are composed of extremely small particles call atoms
2) Atoms of a given element are identical in size, mass, and other properties. Atoms of different elements differ in size, mass and other properties
3) Atoms cannot be subdivided, created or destroyed
4) Atoms of different elements can combine in simple whole number ratios to form chemical compounds
5) In chemical reactions, atoms are combined, separated, or rearranged
#2-atoms are not identical in mass (isotopes), and #3- atoms can be created or destryed (fusion and fission)
Which of Dalton’s Atomic Theory are not true?