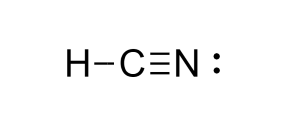CHEM 377: Drugs and Poisons Final Exam
1/84
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
85 Terms
Structure Activity Relationship
which groups are responsible for binding/effect of drug? Can be explored with isosteres
isosteres
synthesized alternative structures which maintain similar group size but may create a change in electronic availability, e.g. change in H bonding or electronegativity
bioisosteres
synthesized alternative structures which maintain similar group size but may create a change in electronic availability, e.g. change in H bonding or electronegativity but have similar biological activity e.g. similarly metabolized as original structure
substituent variation
keeping structure mostly the ssame but varying position or length (e.g. lengthening a carbon chain or moving the group around the phenyl ring)
structure extension
adding more groups to reach a new area in a binding pocket (e.g. make another H bond or reach another vdw region)
chain extension/contraction for epitopes
recall epitopes are linked by a carbon chain; changing the length of the chain
ring expansion/contraction
making a ring larger or smaller (usually 5-7 are stable)
aromatic ring variation
changing the aromatic ring, usually making it a pyridine
ring fusion
adding a fused ring, usually 5 or 6 membered ring
simplification
getting rid of any nasty middle parts that are difficult to synthesize and keeping the active groups
simplification of chirality
getting rid of any chirality by changing the molecule to be symmetric (evening out groups, changing the chiral carbon to a nitrogen)
rigidification
use double bonds, esters, amides, or ring formation to make sure certain groups have a certain position.
conformation blockers
sterically prevent a rotational conformation that is less favorable
magic methyl effect
conformation blocking with just one methyl! One methyl can make big changes
multi target drugs
...have multiple targets. Chain 2 drugs together (hetero/homo-dimeric)
hybrid drugs
2 drugs in a combined molecule since og drugs were similar enough scaffolds to combine
scaffold hop
same active groups, different scaffold: patent busting and companies
optimization of promiscuous drugs
drug may be selective for two target proteins, optimize its interactions with just one
optimizing polarity
change side groups to make more polar/change pKa. -->use bioisosteres
Steric Shield
method of metabolism resistance. Involves putting a group in the way of metabolic enzyme pathway (e.g. tBu by an ester to prevent hydrolytic esterase activity)
Bioisostere electronic effect
using bioisostere may prevent recognition of the motif, i.e. cannot be broken down by enzyme because looks different electronically (e.g. replace beta carbon in a an ester with a nitrogen to make resonance structure which would resist metabolism)
metabolic blockers
changing the group to something that can't be metabolized (i.e. a fluorine isostere)
group shift
method of metabolism resistance. change position of a metabolically active group
aromatic ring variation
metab. resistance: change to a pyridine or add an EWG like CN)
introduce a metabolically sensitive group
...add a group like an exposed CH3 or a small unhindered ester
self destruct drugs
drug self destructs regardless of metabolic enzymes (e.g. sensitive to temperature and metabolizes to
targeting tumors
attach drug to a.a. or nucleic acids, or antibodies for tumor
targeting GI tract
make drug very polar/ionic bc then cant enter cell membranes
targeting peripheral nerves
increase polarity a little bit (but not too much since BBB is still kinda greasy
targeting membrane bound proteins
add a carbon chain - hydrophobic, will nestle into membrane and intxt w membrane proteins
reducing toxicity
remove toxicophores
toxicophores
groups that are toxic or metabolized to toxic compounds [ know some examples or toxicophores]
prodrug
drug not in active form when first ; metabolism puts it in active form
prodrug-improve membrane permeability
more lipohilic at first, then more polar after metabolism. E.g. ester to mask Coo-, N-Methyl to mask NH) KNOW EXAMPLES
prodrug-prolong activity
A) Drug as a leaving group; e.g. interacts with gluthathione. B)lipophilic tether with an ester: slow release from fat tissue
prodrug-mask toxicity
e.g. that hydroxyl group causes stomach bleeding; hide with an ester
prodrug-Lower solubility in blood
why? Reduce bad taste, slower release (accumulate in fat cells); e.g. add hydrophobic chain
prodrug-Improved water/blood solubility
too greasy, all goes to fat; increase blood solubility (e.g. add amino acid or phosphate)
prodrug-target drug delivery
put on a group to be metab by bacteria or viral enzymes or low pH (in stomach or bladder)
prodrug-increase stability
e.g. beta lactams interupted from being attacked by intermolecular nucleophile by closing off with a ring structure
prodrug-activated externally
"sleeping agents" activated by UV/light, etc
who chooses disease targets?
companies pick chronic disease in wealthier countries. Researchers/humanitarians pick diseases in poorer countries
Key facets of picking a drug target
Species selectivity, protein selectivity, organ selectivity
multipathway and multitarget drugs in drug design
drugs may interact with different pathways, or interat with multiple targets; consider these also during design (e.g. off target interactions) different pathways in the cell can counteract efficacy of drugs
bioassay
pre-clinical trials; studies not in humans
in vitro (pros/cons)
pros: cheap, 1,000s to 10,000s scale, fewer variables, can find some toxicity, less controversial, many ways to measure; cons: no ADME data
in vivo (pros/cons)
pros: Adme data, closer to humans, can indicate more toxicity; cons: not automated, more controversial, expensive, smaller samples 10s-100s scale, doesn't model human enzymes perfectly in metabolism
natural product screening(pro/cons)
pros: lots of varied structures, potent, orgs use chemicals for defense; cons: hrd to isolate, small quantities, difficult to synthesize
medical folklore ((pro/cons))
pros: can have potent compounds, cons: can give way to quakery and placebos, ineffective, naturalistic fallacy
Me too/me better
drugs from diffferent companies looking at same target
SOSA
Selective Optimization of Side Activites; optimize the side effects of an existing drug to make new drug (e.g. warfarin derivatives)
modifying original ligands
can make an inhibitor, agonist, antagonist (like our drug project)
combinatorial and parallel synth
can be automated; synthesize a small quantity of 100s-10000s of drugs; shotgun approach
in silico (virtual) screening
computer simulations, can screen millions, lots of false positives, requires known PDB structure
serendipity
chance. Yay!
fragment-based discovery
design little epitopes for different binding pockets, attached with a carbon chain
epitopes
small molecules that interact with different parts of a binding pocket
rule of 3
MW<300, HBD<3, HBA<3, logP<3, rot bonds< 3, tPSA<60 A^2 (less than or equal to)
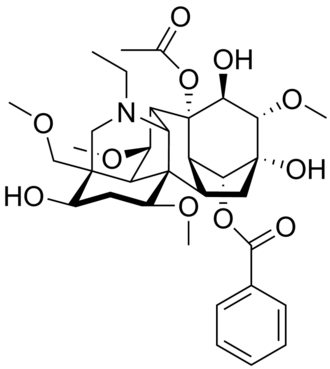
Aconitine
Source: Plants in the X genus (Wolfsbane)
Death occurs due to cardiac arrest and/or respiratory paralysis.
Aconitine Mechanism
Mechanism: (Agonist) Locks sodium-ion channels in the open conformation. Depolarizes membrane potenital, the nerves can’t reset.
Source: Plants in the X genus (Wolfsbane)
Aconitine Structure
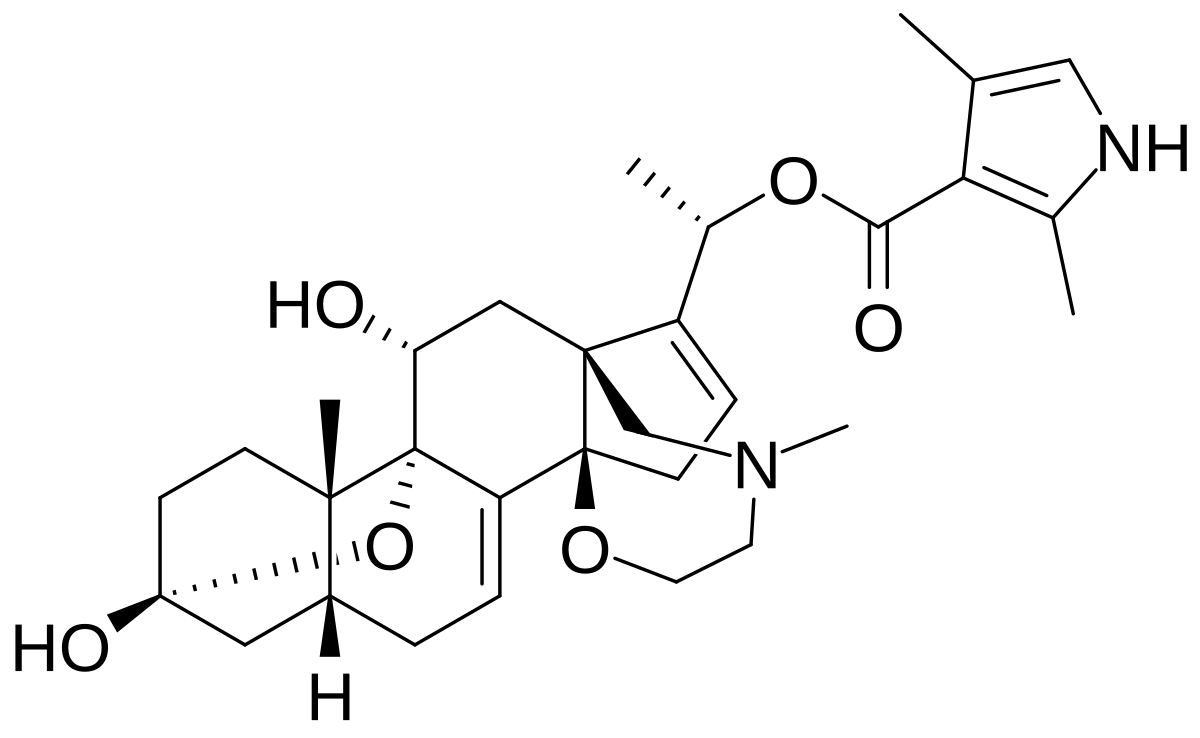
Batrachotoxin
Source: Poison dart frogs (Genus: Phylobates)
Death occurs due to cardiac arrest and/or respiratory paralysis.
Batrachotoxin Mechanism
Mechanism: (Agonist) Locks sodium-ion channels in the open conformation. Depolarizes membrane potenital, the nerves can’t reset.
Source: Poison dart frogs (Genus: Phylobates)
Batrachotoxin structure
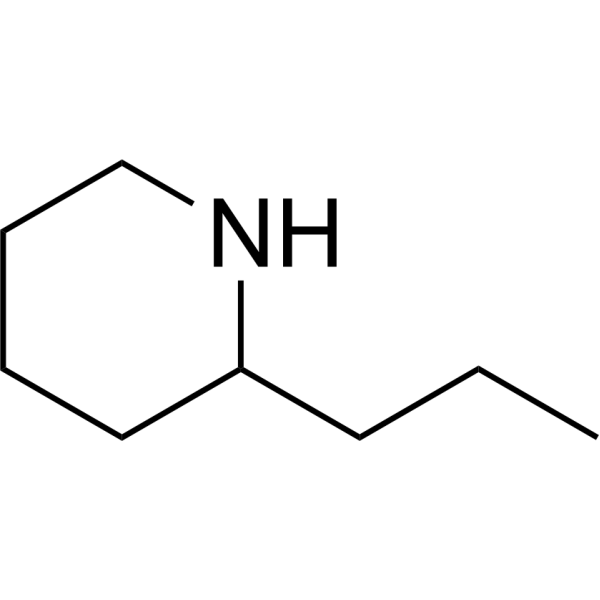
Coniine
Source: Poison hemlock (Xmaculatum) and Yellow Pitcher plant
Death caused by asphyxia due to inability to breathe.
Coniine Mechanism
Mechanism: (Agonist) Locks sodium-ion channels in the open conformation. Binds specifically to the sodium ion channels that respond to acetylcholline or nicotine.
Source: Poison hemlock (Xmaculatum) and Yellow Pitcher plant
Coniine Structure
Tetrodoxin
Source: Marine sources: Pufferfish, octopi, squid, horshoe crabs, some flatworms and ring worms.
Produced by symbiotic bacteria
Tetrodoxin Mechanism
Mechanism: (Antagonist) Blocks sodium ion channels. Nerves unable to signal (loss of feel). Toxic doses cause paralysis of heart and diaphragm.
Tetrodoxin structure
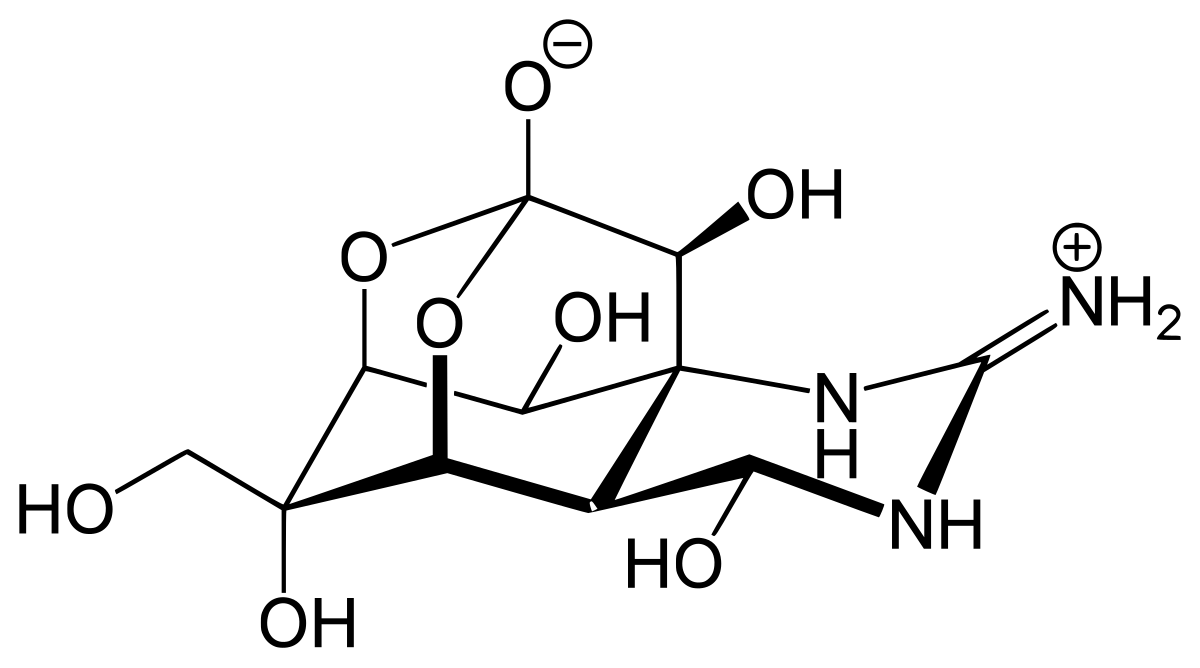
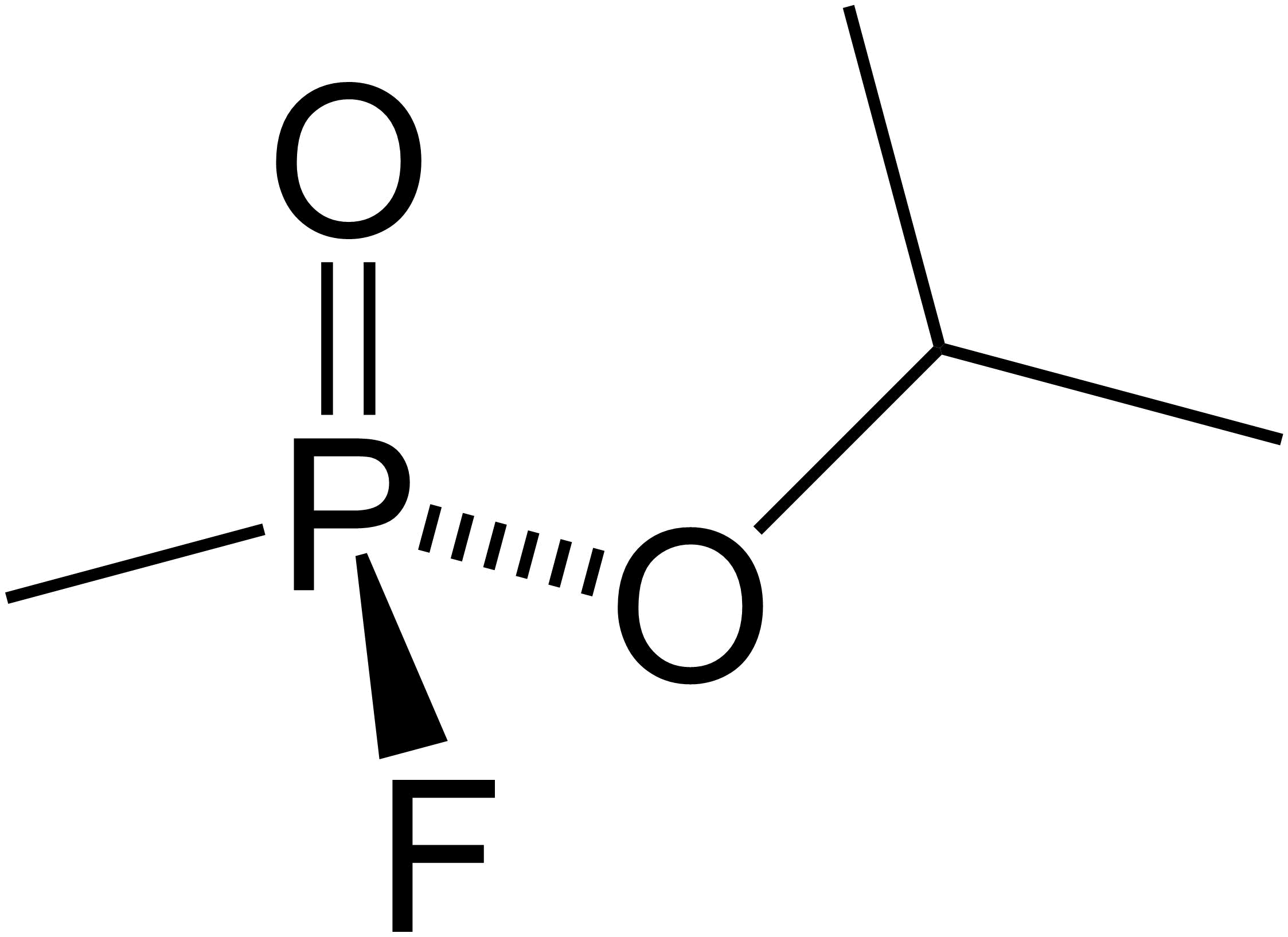
Sarin
Source: Based on a pesticide called Tabun. Used by Germans in WW2
Death caused by asphyxia due to inability to control the diaphragm.
Sarin Mechanism
Mechanism: Irreversible inhibitor of AChE. Leads to buildup of acetylcholine, causing continuous neuron activation.
Sarin structure
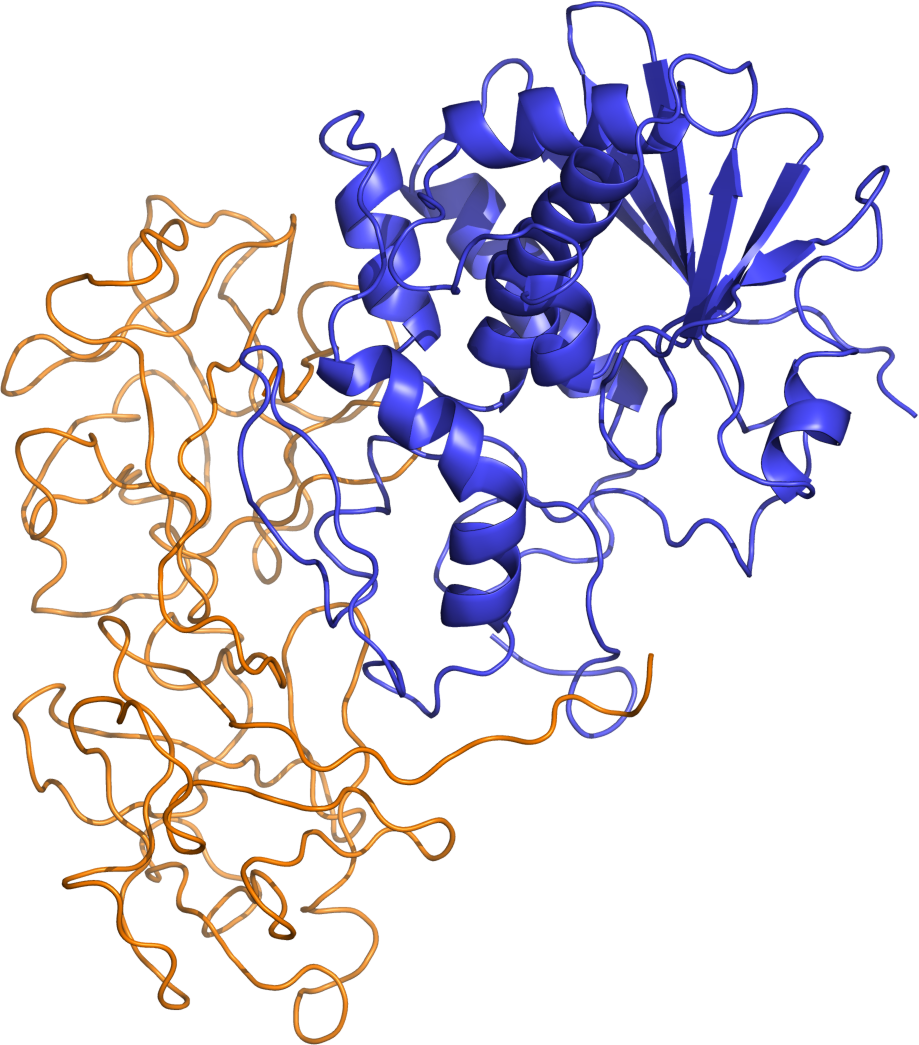
Ricin
Source: Isolated from Castor beans (X communis). The A-chain of the protein heterodimer held together with a disulfide bond is the active chain responsible for toxicity.
Death occurs by shock and organ failure.
Ricin Mechanism
Mechanism: A-chain cleaves adenin in 28S rRNA. A structural component of the ribosome needed for function (ribosomal RNA). One A-chain can cleave these adenine residues in 1500 ribosomes per minute.
Ricin structure
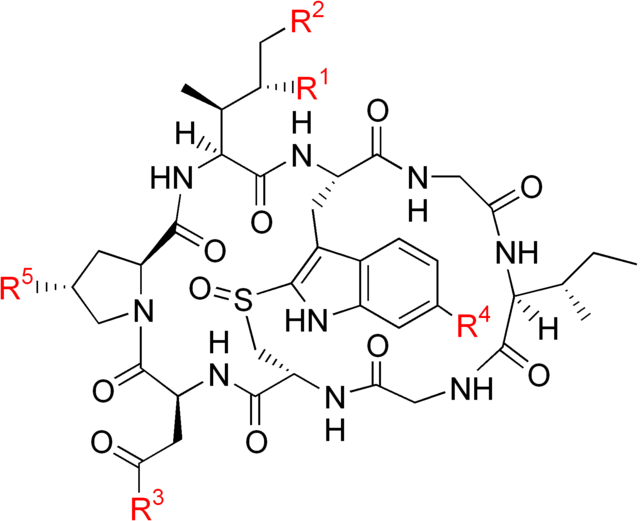
Amatoxin
Source: Isolated from mushrooms (X, Galerina, and Lepoita) aka death cap or paddy straw. Related compounds: α-Amanitin, β-Amanitin, γ-Amanitin
Death occurs several days after ingestion typically due to organ and kidney failure
Amatoxin Mechanism
Mechanism: Interferes with the movement of the “bridge helix” in RNA polymerase II. Movement of the bridge is required for translocation. α-Amanitin binding reduces nucleotide rate from several thousand per minute to <10 per minute.
Amatoxin structure
Mustard Gas
Source: European chemists in late 1800’s. Germany WWI. Results in bullae (fluid-filled blisters)
Initial effects are intense itching and blistering (chemical burns). Death occurs due to severe burn injuries.
Mustard Gas Mechanism
Mechanism: Targets DNA. Alkylating properties make them strongly mutagenic (DNA altering) and carcinogenic (cancerous)
Mustard Gas structure
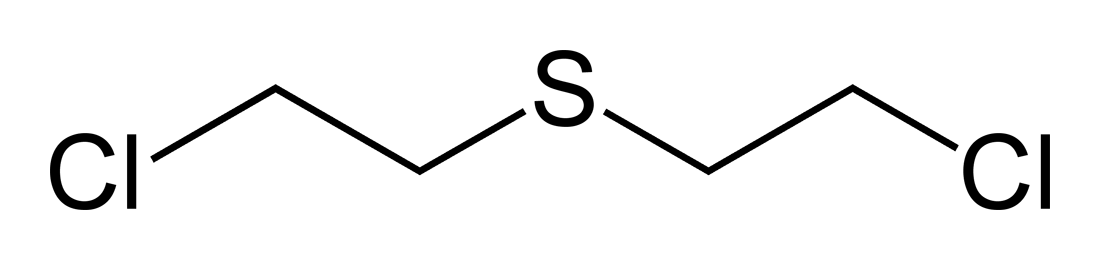
Zyklon B
Source: Cyanide-based pesticide. Germany, Jew execution, 1942
Acute exposure leads to cardiac arrest.
Zyklon B Mechanism
Mechanism: Targets the Electron Transport Chain/ATP Synthesis. Hydrogen cyanide released once canister is exposed to air. Binds the heme in cytochrome c oxidase: Halts electron transport chain.
Zyklon B structure