chem20212 - electrochemistry
1/73
Earn XP
Description and Tags
electrochemistry, quantum, electronic structure
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
74 Terms
equation for molarity
c = mol dm-3
V = dm3
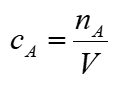
molality equation
m = mol kg-1 M = molar mass kg mol-1
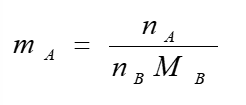
mol fraction equation
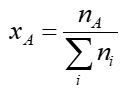
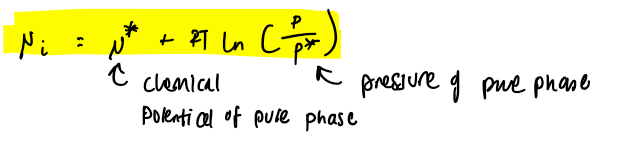
chemical potential equation of an ideal gas in pure phase
μ = J mol-1 G = J
chemical potential is the molar gibbs of the pure phase at constant p,T if it behaves ideally
chemical potential and gibbs free energy at equilibrium
ΔG = 0 at equilibrium therefore chemical potential is constant
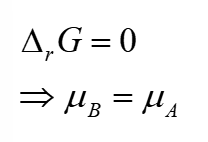
chemical potential of a real gas - expansion at constant T
μ = J mol-1 p = Pa = J m-3
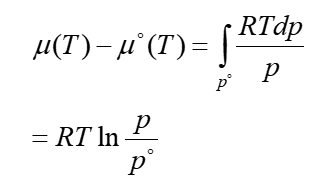
total gibbs energy before mixing ideal gases
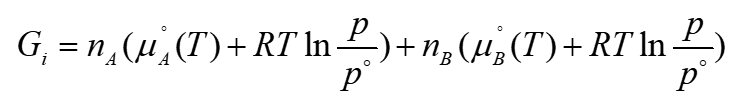
total gibbs energy after mixing ideal gases
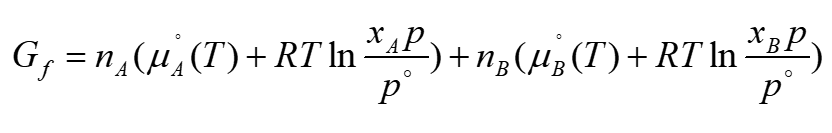
total Gibbs energy of mixing ideal gases eq. how does this prove spontaneity
shows that mixing ideal gases is always spontaneous because ΔG = -ve
xi < 1 and nRT = +ve → -ve + +ve = -ve

Gibbs energy of mixing ideal gases at not equal pressure eq
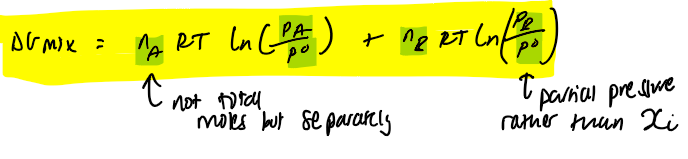
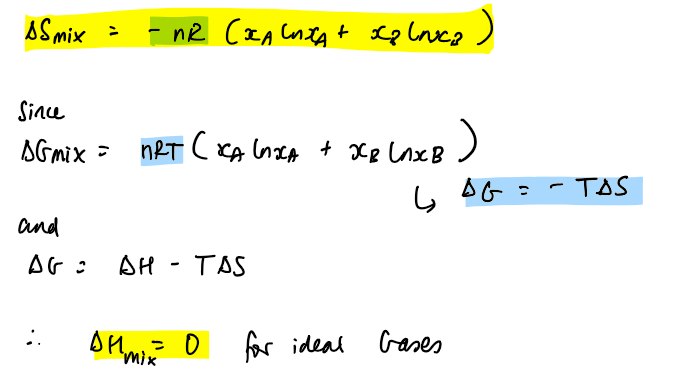
total entropy of mixing
the bottom part is the derivation
free gibbs energy equation
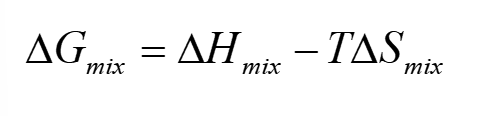
chemical potential of real gases
φ = fugacity → its an effective pressure, correcting for non ideality
deviation between chemical potential of ideal gases and real gases is due to attractive Van Der Waal interactions
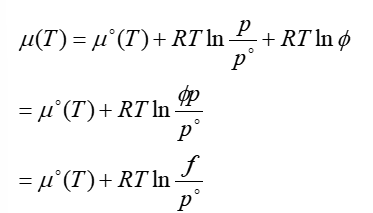
chemical potential of ideal solutions
raoults law eq and argument
argued that mol fraction in solution reduces vapour pressure of certain mixtures from the pressure in pure phase. shown in eq
he takes the assumption that components in solution will behave like ideal gases → mixing of gases is driven by entropy so ΔH mix = 0 → no change in interactions before and after mixing
this will only be approximately accurate if A is surrounded by A ( A being solvent)
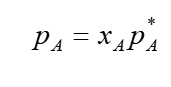
chemical potential of solution according to Raoult’s law
assumption is made that components in solutions behave like real ideal gases
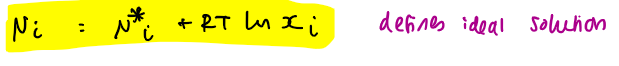
Henrys law
K = empirical constant for pure component B
about ideal dilute solution
implies interactions before / after mixing are different → will be approximately valid when B is surrounded by other molecules (A) . B being the solute
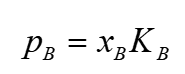
what does it mean by colligative properties
important properties of liquid solution → Tm, Tf, b.p elevation
this is dependent on the amount of solute not its identity
what are the two arguments for why chemical potential in pure phase > actual chemical potential
thermodynamic origin of solution stability
solution formation ΔG = -ve
in pure liquid ΔS is increased by evaporation → more favourable to be in evaporated state
in solution the motion is more random (ΔS is higher than in pure phase) → lower thermodynamic tendency to evaporate
semi quantitative argument
chemical potential eq for ideal solution is μA = μA* + RTlnχA
we know μA < μA* because the latter part of the eq is -ve
we know at equilibrium μA(l) = μA*(g)
if μA is lower in solution, we need higher temp to make it boil and lower temp to make it freeze
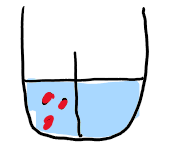
what happens to this over time
diluting the solute is favourable, therefore pressure increases in solution compartment
called osmotic pressure
driven by thermodynamics
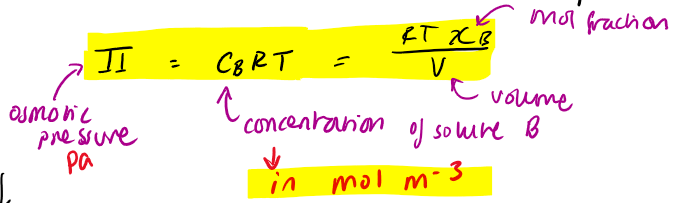
osmotic pressure equation for dilute solution (when xB v. small)
Vm = molar volume
apply this to water purification
if p > Π then water will be squeezed out
change in boiling temperature eq
can switch xB with mB because xB < < 1
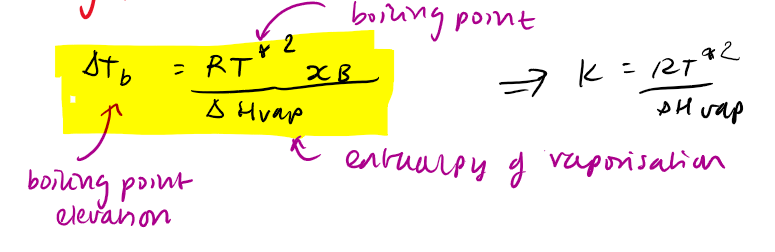
change in freezing temperature eq
can switch xB with mB because xB < < 1
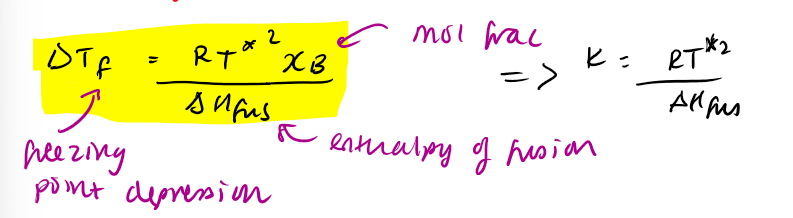
why are alcohols / water and electrolytes not ideal
ideality means interactions before = after mixing
alcohols and water have H bonding when mixed
electrolytes conduct electricity when dissolved → display strong water - ion interactions
compare strong and weak electrolytes
strong - completely dissociate → degree of dissociation is independent of concentration
weak - partially dissociate → degree of dissociation is a function of concentration
what is the definition of the gibbs energy of solvating an ion
the energy required for 1 mol of gaseous ion to become 1 mol of solvated ion
its difficult to measure solvation parameters for individual ions → because other interactions occur

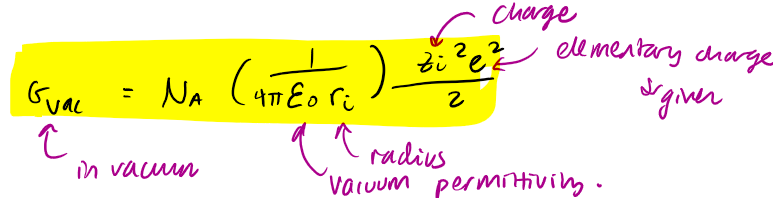
Borns approach to ion solvation - gibbs energy in vacuum
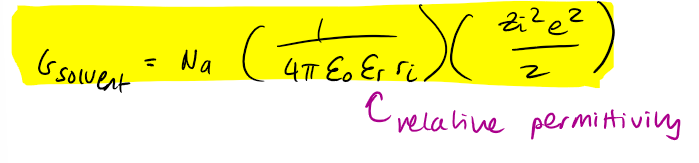
Borns approach t o ion solvation - gibbs energy of solvent
Born’s approach to ion solvation - Gibbs energy of solvation
meaning it’ll always be favourable for ions to solvate
as the εr increases ΔG solvation will become increasingly -ve
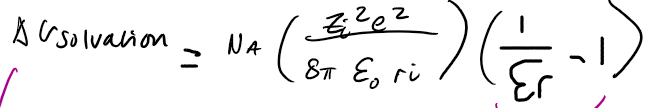
how does transport in electrolytes work
you can force ions to move in a certain direction if you apply a potential difference between two electrodes
this produces an electric field
charge carriers migrate to the oppositely charged electrode → cations to cathode , anions to anode
ohms law
V = voltage
I = current (A)
R = resistance ( Ω ≡ V A−1
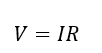
conductance eq
G = conductance
R = resistance (Ω)
k = conductivity (Ω−1 m−1)
A = area (m2)
l = length (m)
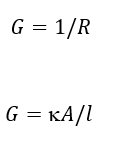
conversion from ohm to siemens

conductance definition
how easily the current will flow through a material
conductivity definition
intrinsic ability of a material to conduct electricity
it increases with concentration increasing. but as conc increases the conductivity will increase by a smaller amount
molar conductivity eq
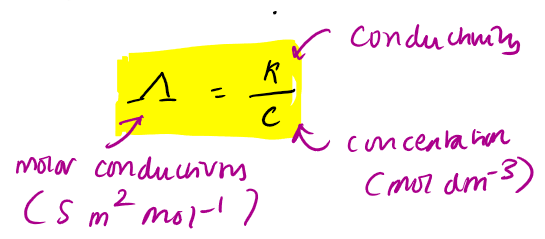
Kohlrauch eq for molar conductivity
molar conductivity decreases as conc increases because interactions with ions. the more interactions the less it can conduct as they are slowed down

limiting molar conductivity eq
Λ0 = limiting molar conductivity, i.e. at infinite dilution (m2 Ω−1 mol−1)
ν = stoichiometric factor
λ = molar conductivity of ionic equivalents (m2 Ω−1 mol−1)
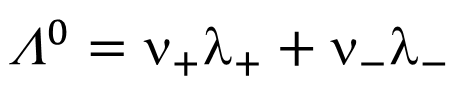
what does the limit of infinite dilution look like on a graph for strong and weak electrolytes
strong → the electrolyte interactions that sloe down the conductivity as conc increases
weak → the dissociation of ions is incomplete and its also a function of conc
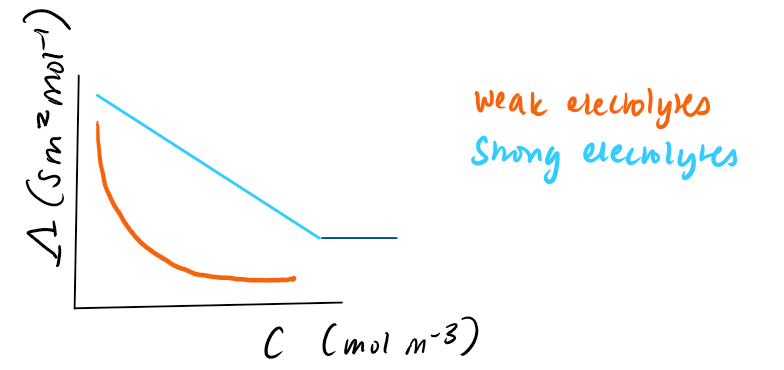
what can you work out with conductivity
measuring the degree of dissociation of weak electrolytes
limiting conductivity values relate to ion mobility
relating ion mobility to effective size of ions in solution → the ion + solvation shell
osmotic pressure equation for non dilute solution (when xB bigger )
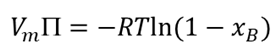
chemical potential of liquid solution that doesn’t behave ideally
γ = activity coefficient
m = molality mol kg-1
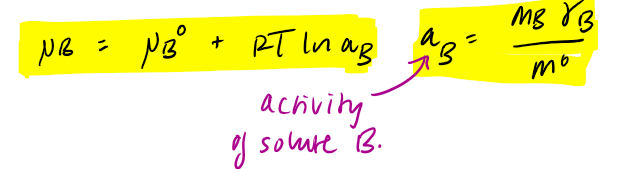
what does the activity of a solute aB tell us
the effective molality of the solute → correcting for solute-solute interaction
when RTlnγB = 0 the solution will be ideal → this would only be the case in very dilute solution
in electrolytes the cations surround the anions forming ionic atmospheres → stabilising the solution
Debye-Huckle law - activity coefficient eq
unitless
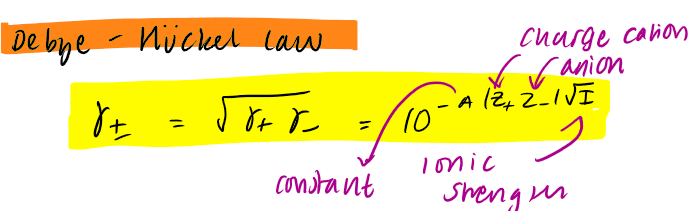
ionic strength eq
unitless
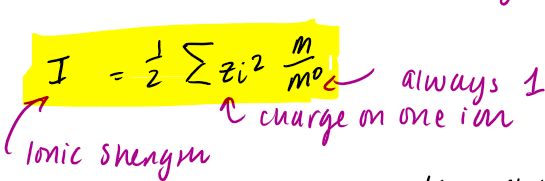
what does the Debye-Huckle law works for and what does it not work for
works for low ionic strength / weak electrolytes
deviates at higher Ionic strength due to ‘excluded volume’
you’d need to modify the DH law to allow for
distance of closest approach → meaning it doesn’t account for the physical size of ions and treats them as point charges - as in the radius is 0
water of hydration bound to ions
ionic liquids - all conc electrolyte no solution
what’s a room temperature ionic liquid
everything is an ion there is no solvent
they’re a liquid because the ions are large AND they don’t pack easily
have bad conductivity
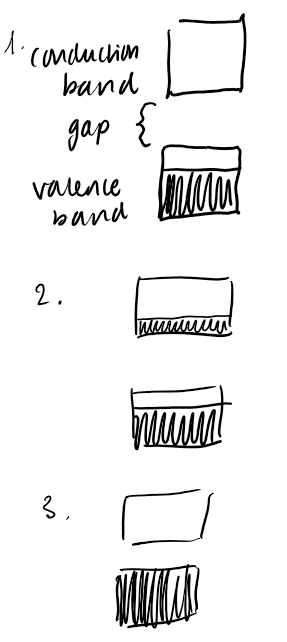
what are 3 types of metals
conductors - band is not completely filled so it has accessible energy states for e-
semi-conductors - band gap is comparable to thermal energy so at certain T , some e- are promotes
insulators - band is full so no accessible states
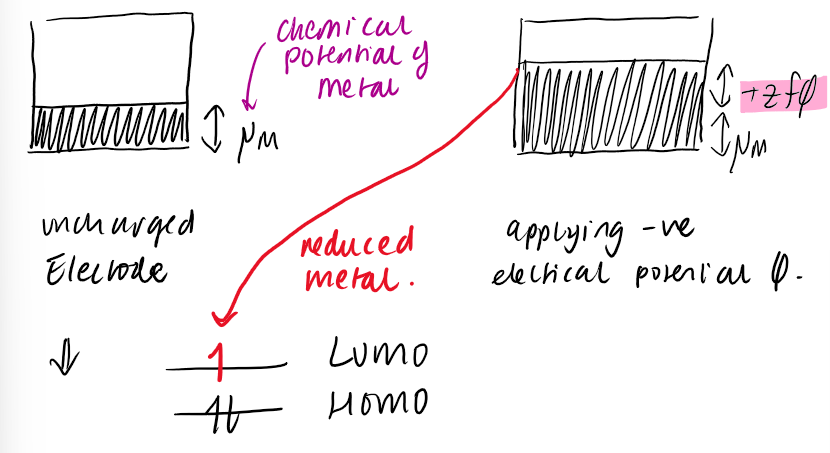
what is Fermi energy
the top of the valence band where all the interactions happen
it can be equated to chemical potential of the e- inside the solid
the energy of the e- can be changed by applying external electrical potential φ
how does e- transfer work on orbitals and on the fermi energy
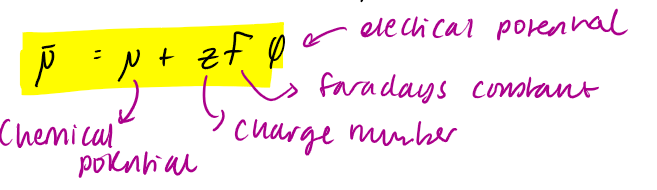
electrochemical potential eq
φ = electrical potential (V)
μ— = electrochemical potential = +ve
electrochemical potential eq of oxidised species

overall electrochemical potential equation that shows the electrochemical potential of the reaction = 0
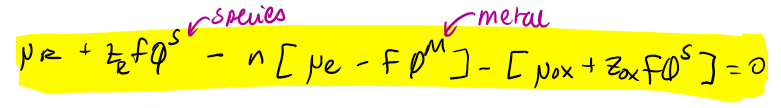
equation where chemical potential eq is substituted in electrochemical potential eq
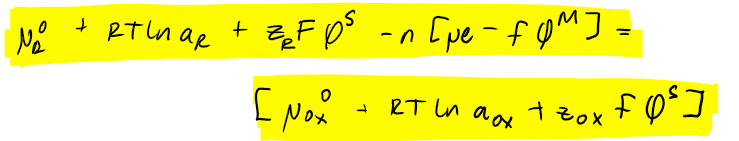
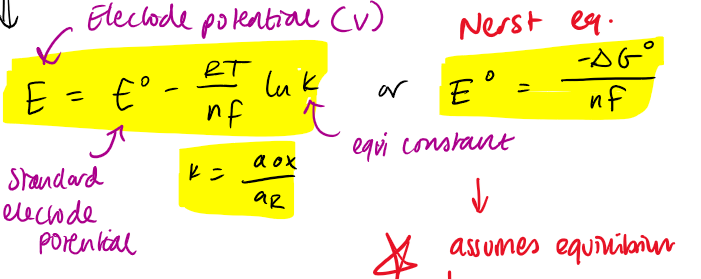
electrode potential + nerst eq
rearranged electrical potential equation that includes G
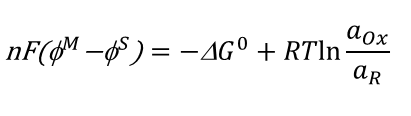
daniel cell eq

what does a Daniel cell look like
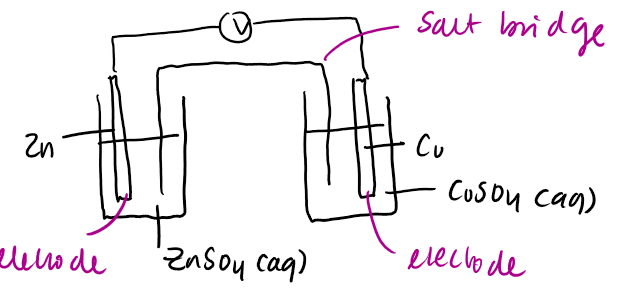
how would you write the system of a cell
oxidation on the left
reduction on the right
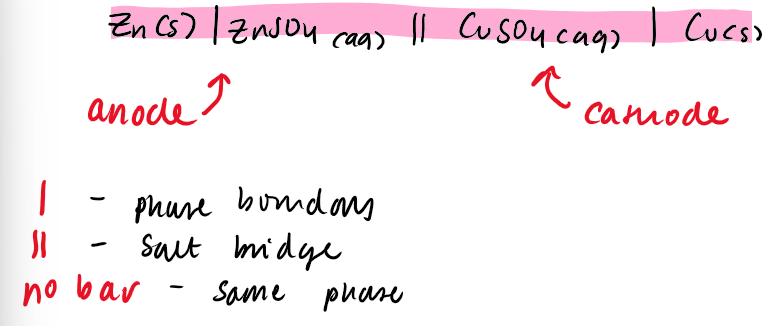
what is the reference redox couple
the Hydrogen electrode (SHE)
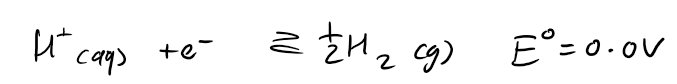
pH eq
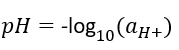
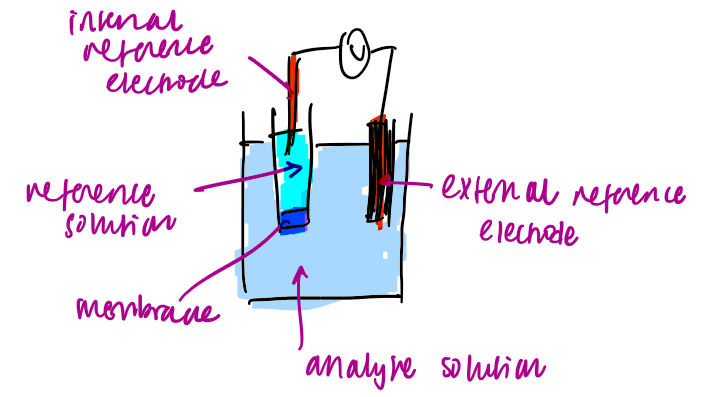
what does an ion selective electrode look like and what are the three phases
the 3 phases
analyte solution
reference solution
membrane
the target ion can go between all three
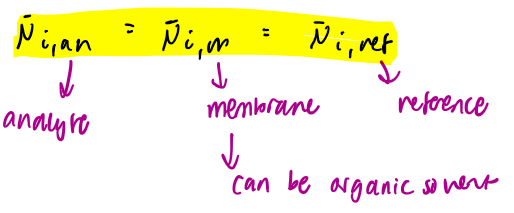
electrochemical potential of analyte in an ion selective electrode eq
ion transfer / electrical potential eq
tells us the electrical potential required to drag ions into the membrane phase
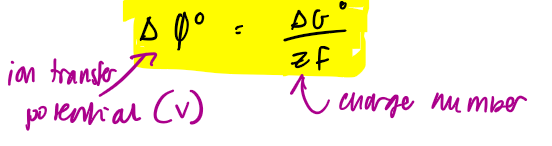
measured electrical potential difference eq
shows us that the difference in electrical potential between the two electrodes φref and φan is proportional to the ratio of activities of the distributing ion in the phases

why store energy electrochemically?
thermodynamic limit on efficiency of combustion energy → electrochemical has a higher limit
greenhouse gas issues from combustion of hydrocarbons
increasing use of renewable energy
what are the 2 methods of storage of electrochemical energy
reversible conversion of chemical energy → electrical energy - secondary battery
irreversible conversion - primary battery ( can’t recharge)
what are the disadvantages of batteries
reactions not readily reversible - Zn2+ tends to form complexes that block contact between electrode and cathode
MnO2 is a poor electrical conductor - need inert carbon to transfer e-
heavy materials used - energy / mass ratio is low
cell voltage is limited to 1.4 -1.7V
what is the cathode and anode reaction on charging in non aqueous batteries
lithium batteries
the cathode and anode flip during discharging
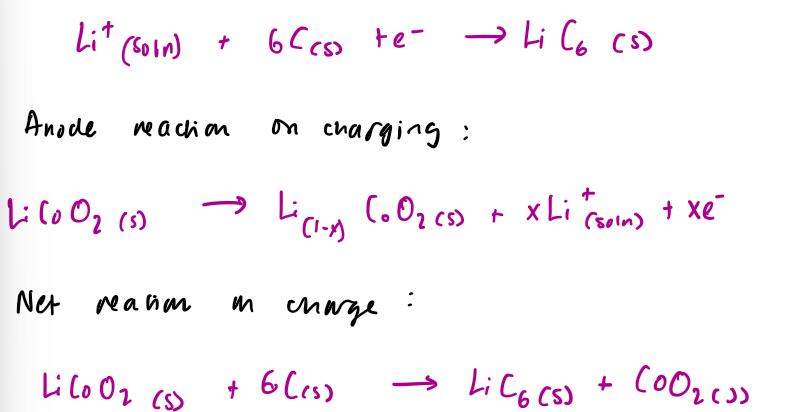
energy density eq for batteries
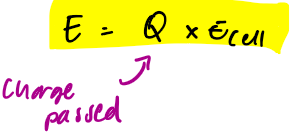
what are ideal battery characteristics
max energy density
reversibility
safe, non-toxic
light environments - use of Li
not reactive in H2O
what are disadvantages of Li batteries
organic solvent required - flammable, toxic
graphite intercalation used for prevention of formation of dendrites - dead weight
use of transition metal oxides - expensive , heavy
poor electrical conductivity
what’s the graphical shape of charge and discharge of Li batteries
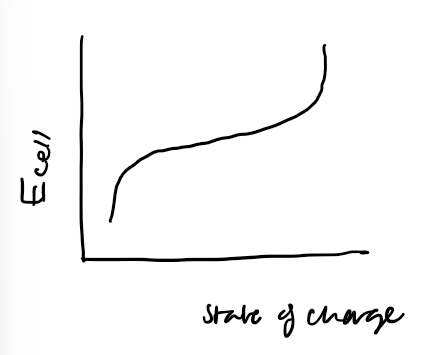
what’s the problem with the Nernst eq
assumes equilibrium
sets a theoretical potential that can be achieved
we need to consider
kinetic loss
ohmic loss
conc loss