AE 279 Energy and Environment Midterm 1.1, 2.1, 2.2
1/95
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
96 Terms
Name the following acronyms.
E
m
Q
S
W
t
T
V
z
d
δ
△
energy
mass
heat
entropy
work
time
temperature
velocity
elevation
small change
small amount
finite change
What is the 1LTD? What is 2LTD?
1st law of thermodynamics (1LTD)
Energy cannot be generated or destroyed
It CAN change form and can be transferred
Egen = 0 and Edest = 0 (an E bal accounts for all energy in a system/process)
2nd law of thermodynamics (2LTD)
Entropy can be generated but not destroyed
It CAN be transferred
Sgen ≥ 0 and Sdest = 0 (an S bal indentifies limits on what is possible for a system/process)
What is the difference between transfers and gen/dest?
Transfers
A quantity crossing the SYS boundary (transferred to a SYS or from a SYS
Can be 0 + -
A - val means assumption is wrong (switch sign)
Gen/Dest
Due to internal processes with a SYS boundary
Can be 0 +
A - val means that there may be a calculation mix
Thermodynamic analysis involves what?
Thermodynamic analysis involves applying balance equations to energy related quantities to an identified system undergoing some process.
What are the three energy storage modes? Label them as macroscopic or microscopic and their notation.
1. Translational kinetic energy (macroscopic) KE
2. Gravitational potential energy (macroscopic) PE
3. Internal energy (microscopic)
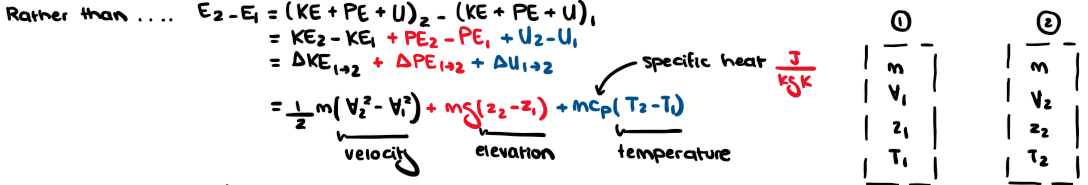
Give an example of an isolated system and how it works.
A cooler!
E2 - E1 = 0 (no heat transfer, no work transfer, no mass transfer)
Energy has rearranged itself. Internal change happens but energy in the system does not change. Internal energy stops when an evening out/equillib happens.
BUT entropy changes: it tells us what's possible as time moves on (accounting system). STILL, energy is not generated or destroyed (irreversible entropy).
There are two types of systems. What are they? Compare and contrast.
Control volume (CV)
control volume = specified region = open system
Mass may cross the boundary
Energy may cross the boundary (heat, work)
Usually, the boundary is fixed spatially
Control mass (CM)
control mass = specified matter = closed system
Mass/matter does NOT cross the boundary
Energy may cross the boundary (heat, work)
Spatial location of boundary may change
Can still change shape!
What is work transfer and what are is its denominations? What is heat transfer and what are its denominations? Compare and contrast.
Work transfer (W = work, Ẇ = rate of work transfer, δW = small amount of work, Wel = electric work, Wsh = shaft work)
Is associated with the application of force through a distance (pushing, pulling, twisting)
A transfer of organized energy
Typically measurable
No entropy transfer present with work
Heat transfer (Q = heat, Q̇ = rate of heat transfer, δQ = small amount of heat)
Is associated with temperature difference- net heat transfer occurs in direction of decreasing temperature
A transfer of disorganized energy
Impractical to measure directly. INSTEAD, it is inferred using other measurements and balance eqns
Entropy is transferred with heat
Name the following nomenclature
bal
CM
CV
dest
gen
SF
SS
SYS
SURR
TD
1LTD
2LTD
PCom
rms
balance
control mass
control volume
destruction
generation
steady flow
steady state
system
surroundings
thermodynamics
1st law of thermodynamics
2nd law of thermodynamics
principle of conservation of mass
root mean square
Thermodynamics involves systems, surroundings and boundaries. Define each and how they relate to one another.
System (SYS): region or matter being considered
Surroundings (SURR): everything outside the system
Boundary: seperates SYS from SURR. Specifying the boundary defines the SYS.
What are some other relevant modes of energy (other than translational kinetic, gravitational potential and internal)?
Elastic potential energy (macroscopic)
Rotational kinetic energy (macroscopic)
Chemical potential energy
Nuclear binding energy
If a process does not cause a change in energy stored by a particular mode, it does not need to be included in the eqn.
What is PCom?
Mass is conserved (ie not created or destroyed)
mgen = 0 and mdest = 0 (an m bal accounts for all mass in a system or a process)
What is entropy? What are some examples?
Entropy is a measurement of disorder. It helps explain why physical processes go one way and not the other.
Examples: why ice melts, why air leaks out a punctured tire, why cream dissipates in a cup of coffee.
These states have more dispersed states than the originals, therefore nudging them towards higher entropy. This is because entropy is statistically more probable.
What is an isolated system?
An isolated system is when a CM has no interactions (heat or work) between the SYS and its SURR.
How does entropy work?
The more energy a solid has, the hotter it is. This means it has more energy. Low entropy means concentration of energy is less spread out and so, higher energy means a more spread out configuration.

What are two notable forms of energy transfer?
Heat and work
What does the dot on top of a letter mean?
X flow rate
For PV = mRT, is T in terms of °C or K?
K
How does temperature relate to energy?
Temperature relates to disorganized microscopic energy of molecules/particles.
What are some examples of microscopic forms of energy?
Microscopic…
translational
rotational
vibrational
energy
True or False. For TD analysis, we work with atmospheric temperature.
False. For TD analysis, we work with absolute temperature.
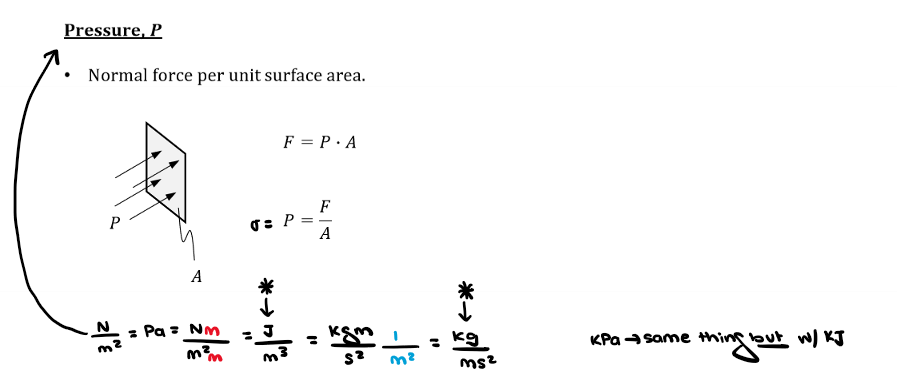
What is pressure?
Pressure is normal force per unit surface area.
True or False. At a point in a fluid at rest (no bulk motion), the pressure is the same irrespective of the direction of measurement.
True! If not balanced/not the same, fluid is moving.
What is the relationship between pressure and temperature? Make some correlations.
As pressure increases, there needs to be a higher temperature for the fluid to boil.
As pressure decreases, there needs to be a lower temperature for the fluid to boil.
What is gauge pressure? How do you get gauge pressure?
Gauge pressure is absolute pressure. It measures the difference between local atmospheric pressure and pressure within the vessel.
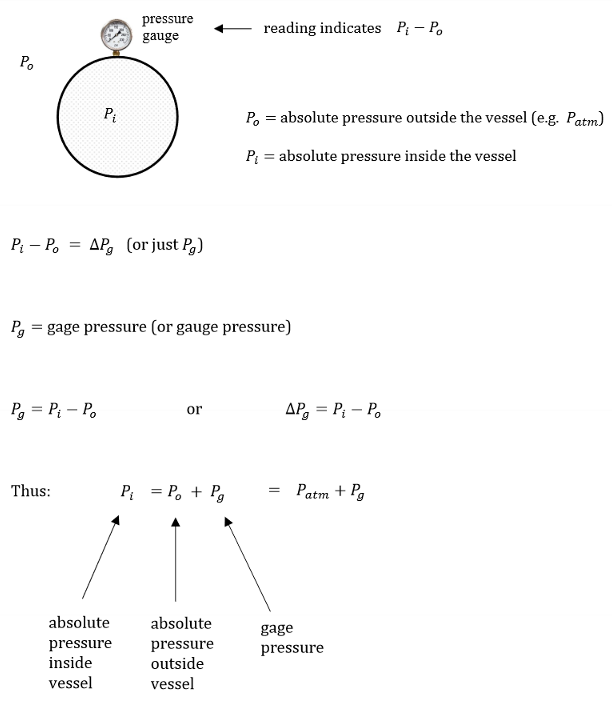
What is a vacuum gauge?
A vacuum gauge measures how much lower the pressure inside a vessel is compared to the pressure around it.
Pi = P0 - Pv
where Pv is the vacuum pressure
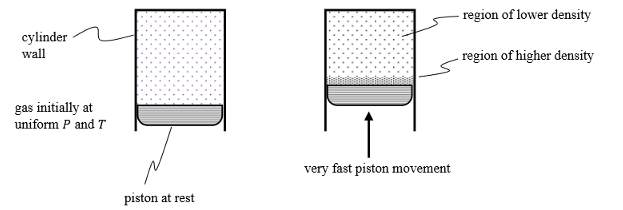
Give three examples on how density can vary locally within a substance.
Density of air decreases with altitude
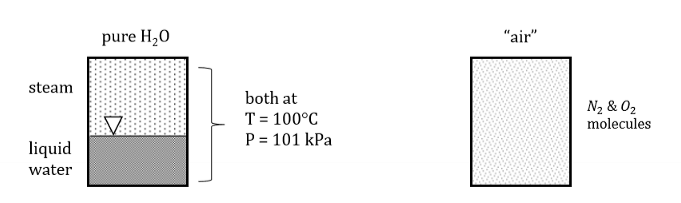
Vessel containing pure H2O in 2 phases
Gas being compresses in a piston and cylinder (see image)
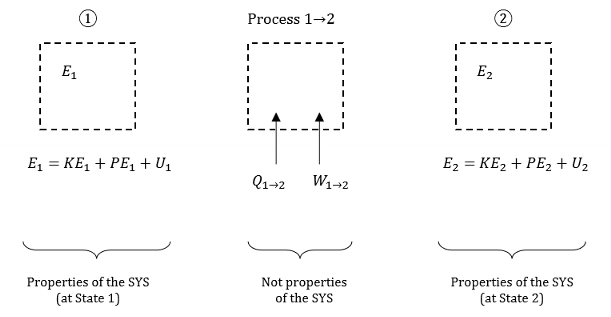
What is a property?
A characteristic that provides information about the state of a system.
A property is assessed at a specific moment in time and provides information about the state of the system at the moment of assessment ONLY. It does not provide information about the history or future of a system.
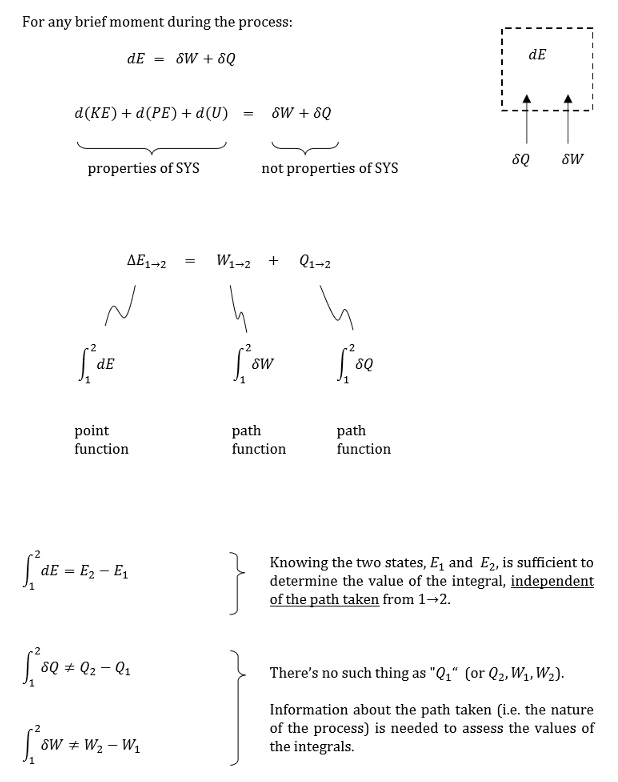
Give examples of properties and those of not properties.
Properties
kinetic energy
potential energy
internal energy
Not Properties
work
heat
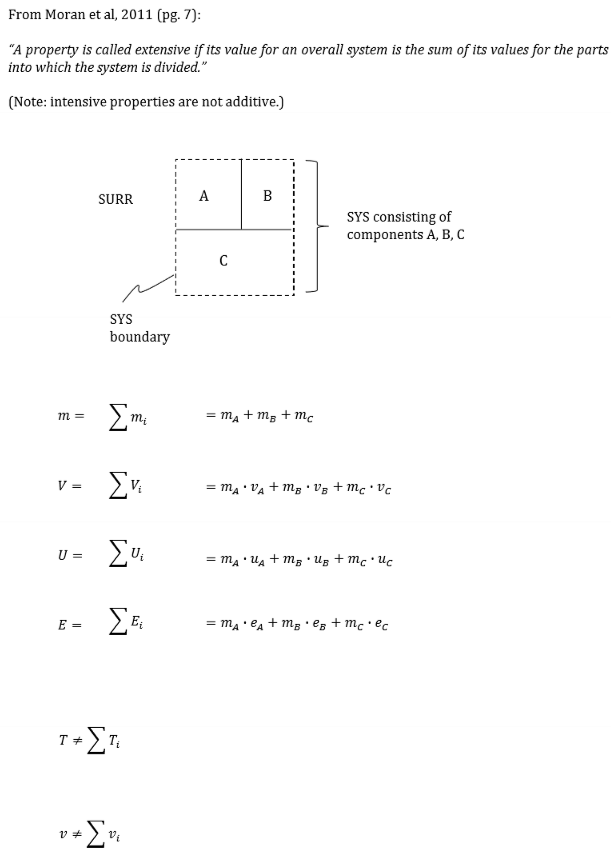
Compare and contrast intensive and extensive properties. Give a few examples of each.
Intensive: Do not provide an indication of the quantity of matter involved.
T = temperature
P = pressure
v = specific volume
ke = specific kinetic energy
u = specific internal energy
h = specific enthalpy
s = specific entropy
Extensive: depend on the quantity of matter
m = mass
V = volume
KE = kinetic energy
U = internal energy
S = entropy

What does specific mean when it comes to energy?
Indicates that quantity is per unit mass
Generally in terms of nomenclature, how is extensive and intensive differentiated?
Intensive = lowercase
Extensive = uppercase
True or False. Intensive properties are additive.
False. Intensive properties are not additive. Extensive properties are additive!
Compare and contrast measurable and calculated properties. Give examples of each.
Measurable (observable): Straightforward to measure if suitable instruments are available.
T = temperature
P = pressure
m = mass
L = length
A = area
V = volume
ν = translational speed
ω = rotational speed
Calculated (inferred)
Not directly measured, but calculated/inferred (based on measurable properties)
pe = gz
keT = ½ V²
u
h
s
PE = mgz
KET = ½ mv²
Do we use macroscopic or microscopic properties for engineering thermodynamics? Why?
We apply relatively simple models of bulk behaviour of substances based on macroscopically observable properties.
We recognize that substances contain microscopic particles but we work at a scale reasonable at a continuum.
What is statistical thermodynamics?
Statistical thermodynamics looks at engineering thermodynamics at a microscopic view (behaviour of molecules and atomic particles such as velocity, trajectory and molecular interactions)
Used to explain behaviour of matter based on probability (due to the intense number of particles involved).
What is a pure substance and how do we apply that to thermodynamics?
A pure substance is a form of matter with a definite and constant composition, meaning it contains only one type of particle throughout
We treat some substances as “pure” even though they may have some trace contaminants. Separate phases may be present in pure substances. Even, homogenous mixtures (like air) can be modelled as pure substances despite having different chemical species.
Define phases.
Phases refer to a quantity of matter that is homogenous throughout both chemical compositions and physical structures.
What is it called when a system contains more than one phase?
A mixture.
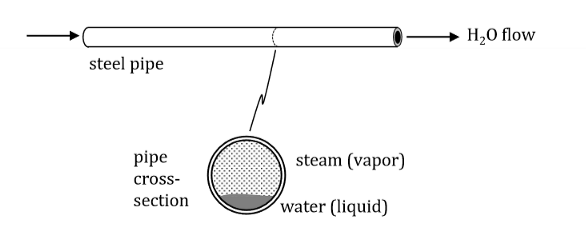
Define moving boundary work.
The resultant of a force applied at a fluid boundary that causes the fluid molecules at that location to move aka a transfer of energy as work.
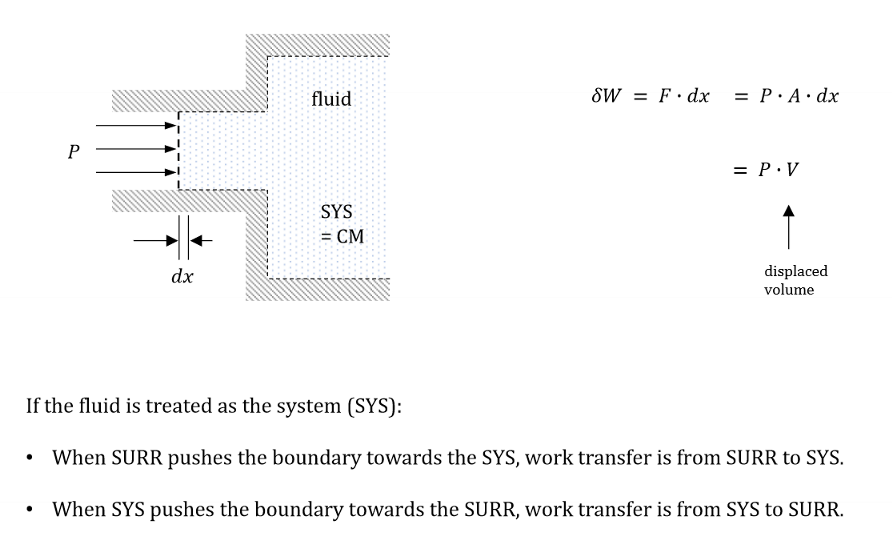
Fill in the blanks.
When SURR pushes the boundary towards the SYS, work transfer is from ____ to ____.
When SYS pushes the boundary towards the SURR, work transfer is from ____ to ____.
SURR to SYS
SYS to SURR
What is a simple compressible substance? What does it entail?
A substance for which the only reversible work mode is moving boundary work.
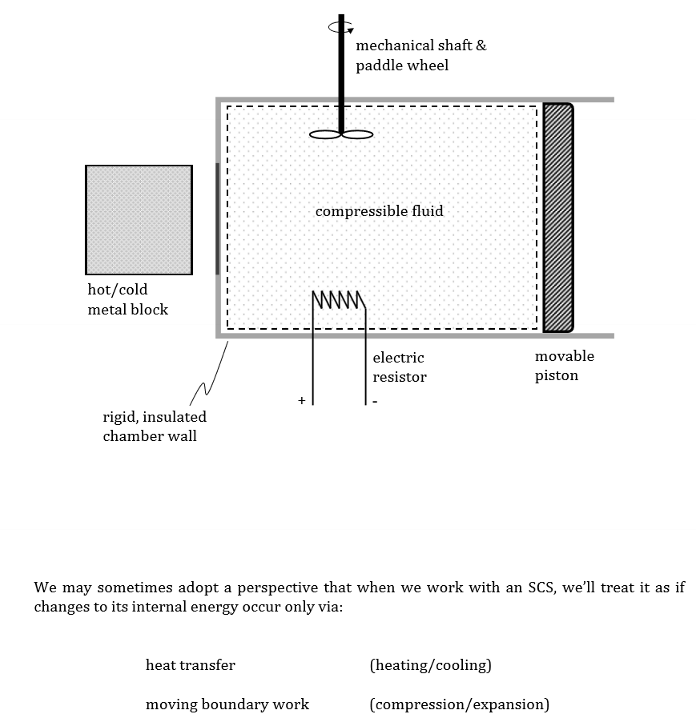
What is heat transfer?
E transfer that occurs due to a differential in temperature (from higher to lower T)
What are the three heat transfer modes? Define each.
Conduction: Diffusion of heat in a stationary medium.
Convection: HT involving movement of a fluid (liquid, gas). MAY be treated as a mass transfer in TD.
Radiation: Heat transfer by photons or electromagnetic waves.
What does it mean when we say two systems are in thermal contact? What is another way of saying this?
Thermal contact = thermal communication
When two systems are in contact with each other when at least one pathway is present for heat transfer between them.
Two systems at the same T and in thermal contact will experience…
zero net heat transfer
Two systems are said to be at the same T (ie in thermal equillib) if they are…
in thermal contact but experience not heat transfer
What is the zeroth law of TD?
If two objects are each in thermal equilibrium with a third object, then they are also in thermal equilibrium with each other.
Third system could be a thermometer!
What is an unintentional heat transfer also called?
Unintentional heat transfer = leak
When does heat transfer occur?
When there is a heat transfer pathway AND temperature difference.
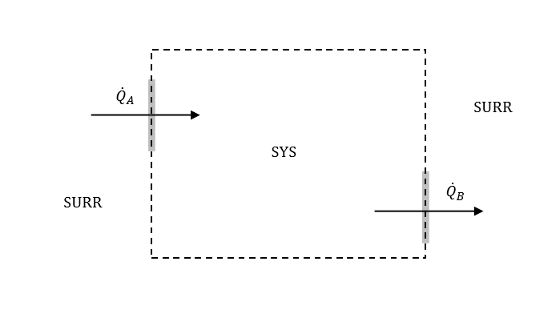
What is an adiabatic process/adiabatic system?
Adiabatic means without heat transfer.
For an adiabatic process, HT between SYS and SURR is negligible (approx 0).
Why do we say externally adiabatic and not just adiabatic?
Externally adiabatic means that there is negligible heat transfer between SYS and SURR. There CAN be HT within the SYS!!
Define internal equilibrium.
A thermodynamic system is internal equilibrium when it is in a state of internal balance.
When a system is not in internal equilibrium, some properties may not have a clear definition (ie P and T)
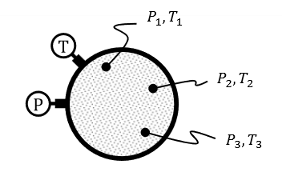
What are the four types of equilibrium?
Thermal: Uniform T. No T gradients to drive heat transfer within the SYS
Mechanical: Forces are in balance. No P gradients to drive macroscopic fluid motion.
Chemical: No differences in chemical composition. SO no concentration gradients.
Thermodynamic: Everything is in balance! No T, P, etc gradients to drive change
^If isolated (aka unable to interact with SURR), SYS would not experience macroscopic change.
Why is internal equilibrium an important concept for TD?
Need to know whether at internal equilibrium to know whether we can apply eqns of state such as ideal gas law, p-h diagrams, steam tables.
What is internal energy?
Internal energy is the energy possessed by a system/substance via microscopic (disorganized/random) modes of energy storage.
What is disorganized KE and PE associated with?
Disorganized = microscopic
Disorganized KE is associated with molecular motion (sensible energy associated with T)
Disorganized PE is associated with intermolecular bonds (latent energy associated with phase changes SO binding forces between adjacent molecules)
Give 7 examples of microscopic modes of energy storage.
Molecular translation
Molecular binding
Molecular vibration
Molecular rotation
Mass equivalent
Electron translation
Electron spin
Electric dipole moment
Magnetic dipole moment
Intermolecular (strong)
Intermolecular (weak)
Nuclear spin
Nuclear binding
Coulomb binding
External field
Pressure varies with depth in fluid. When are pressures lower and when are they higher in reference to elevations?
Pressures are lower at higher elevations and higher at lower elevations.
For example, atmospheric pressure decreases with increasing elevation (to 0 at the edge of Earth’s atmosphere).
Also, pressure increases with depth in a body of water.
True or False. In a continuous mass of fluid at rest (no bulk fluid motion), pressures are equal at equal elevations.
True!
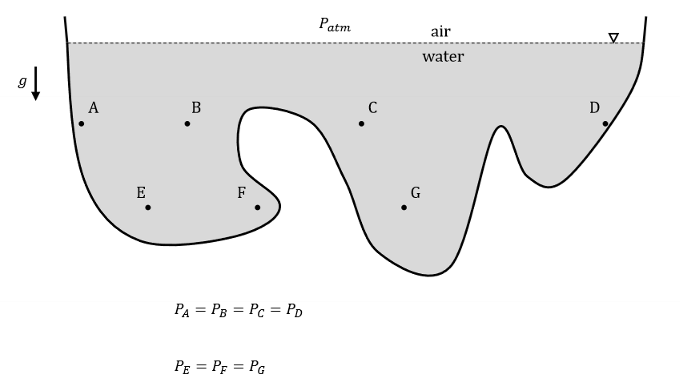
Define absolute pressure. Define absolute temperature.
Absolute pressure is the measure of pressure relative to a perfect vacuum (absolute zero pressure).
Absolute temperature is measured using a scale where zero corresponds to absolute zero, the theoretical temperature at which molecular motion ceases.
Define density.
Density is a measure of how much mass is contained within a given volume of a substance.
Density = mass/volume
Define equilibrium.
Systems in a state of balance where there are no net macroscopic flows of mass or energy, and no tendency for change on a macroscopic scale
Define liquid manometer.
A tool that we use to measure the pressure difference using the height difference of a liquid column.
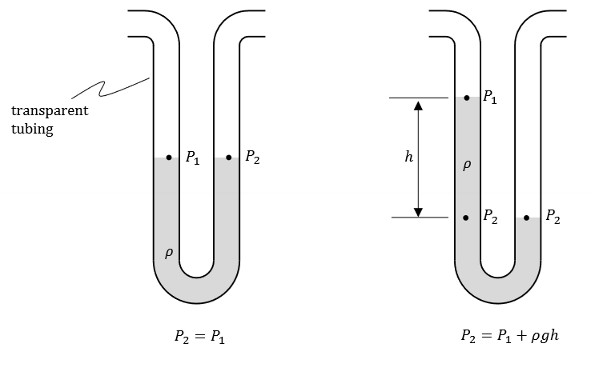
Define gauge pressure.
Gauge pressure is the pressure of a system relative to the surrounding atmospheric pressure. It essentially measures the pressure difference between a system and the ambient air pressure.
Define heat transfer and heat leak.
Heat transfer is the process of thermal energy moving from one object or system to another due to a temperature difference.
Heat leak is an unwanted or unintended heat transfer, often from a warmer area to a cooler one, that can cause energy losses or inefficiencies
Define internal energy.
It's the energy associated with the random, disordered motion and interactions of molecules within a substance.
What is the driving force for a) electric current flow, b) fluid flow and c) heat transfer?
a) voltage difference (delta V)
b) pressure difference (delta P)
c) temperature difference (delta T)
Define a quasi equilibrium process.
The internalized version of a system which remains infinitesimally close to an internal equilibrium equation
(even though the system properties may be changing during the process).
True or False. Quasi equilibrium is a very fast process.
False. It is a very slow process.
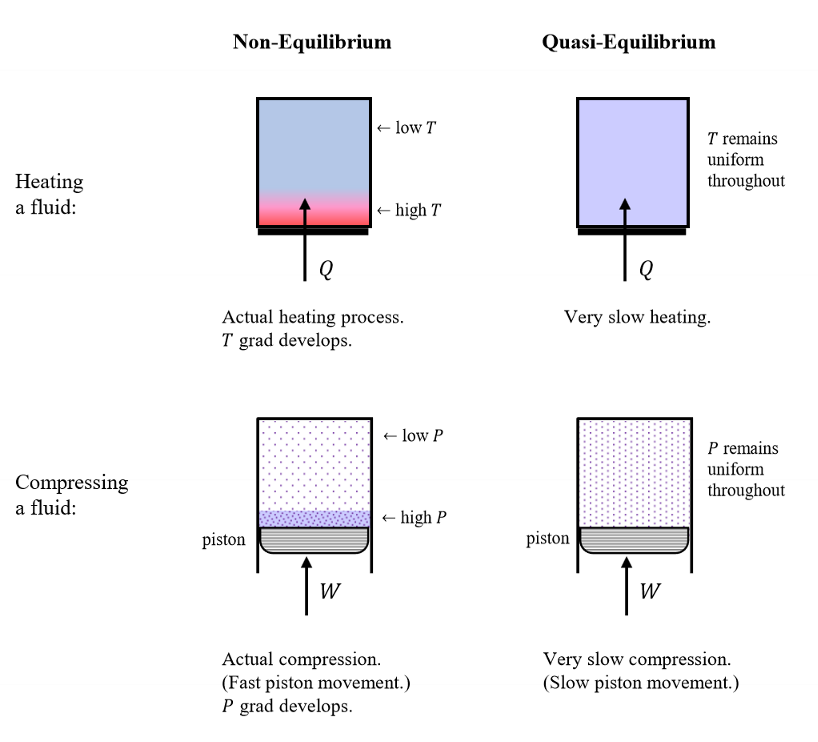
Do internal gradients develop in states of quasi equillibrium?
No. Internal gradients (that could drive macroscopic change) do not develop.
How do you test for internal equilibrium conditions?
Isolate SYS from surroundings (so halt all HT) and then watch to see if there are any changes in macroscopically observable properties. If no changes are present, SYS is in equillib.
Define a process path.
When the state of a system is definable at each moment during the process, we can describe this as a process path.
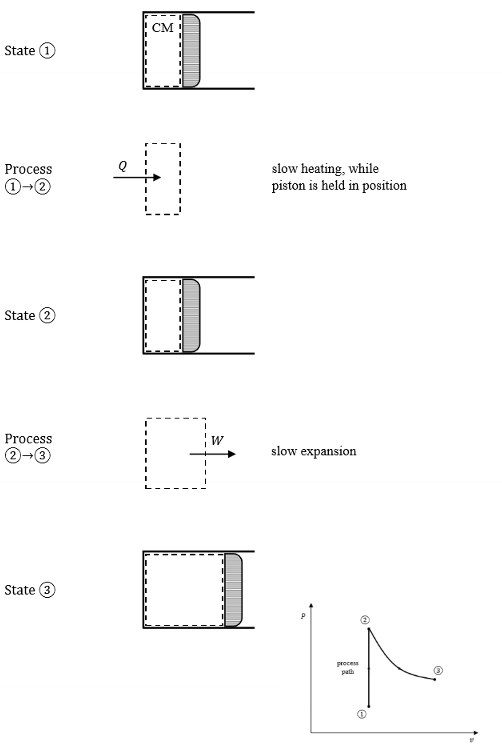
Define a cycle.
A sequence of processes through which the system changes state but eventually returns to its original state.
What is enthalpy? Is it a calculated property?
Enthalpy is a thermodynamic property that represents the total heat content of a system.
h = enthalpy = u + Pv
It is a calculated property.
Total energy of a flowing fluid (θ) is an intensive property. What is it used for?
Is used for calculating the total energy transferred when a mass is transferred (ie flows across) a boundary.
It accounts for energy carried by mass (e) and flow Pv

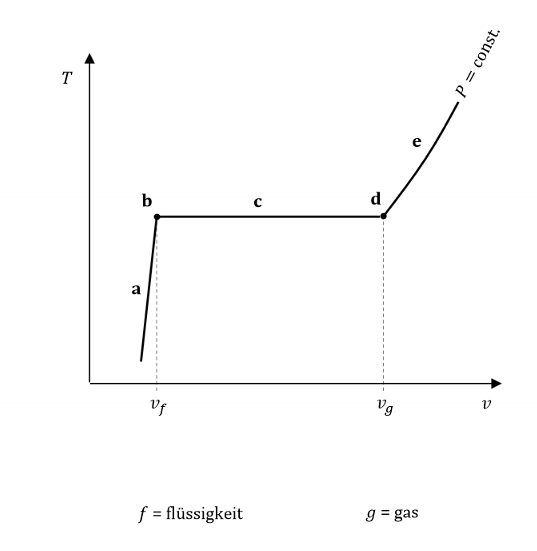
Label each of the points in this diagram

What is a property that indicates the relative amount of each phase?
x = quality (of the mixture)
It tells us the fraction of what is in the gas phase.
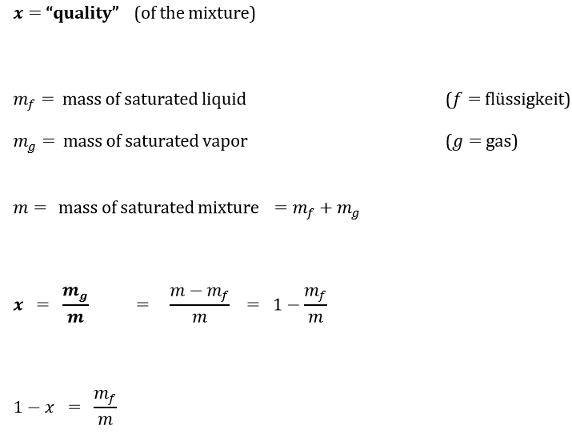
A saturated mixture that is 100% vapour, what is x?
A saturated mixture that is 100% liquid, what is x?
x = 1
x = 0
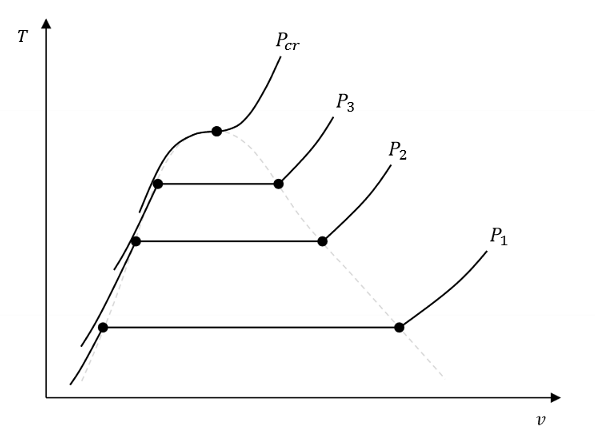
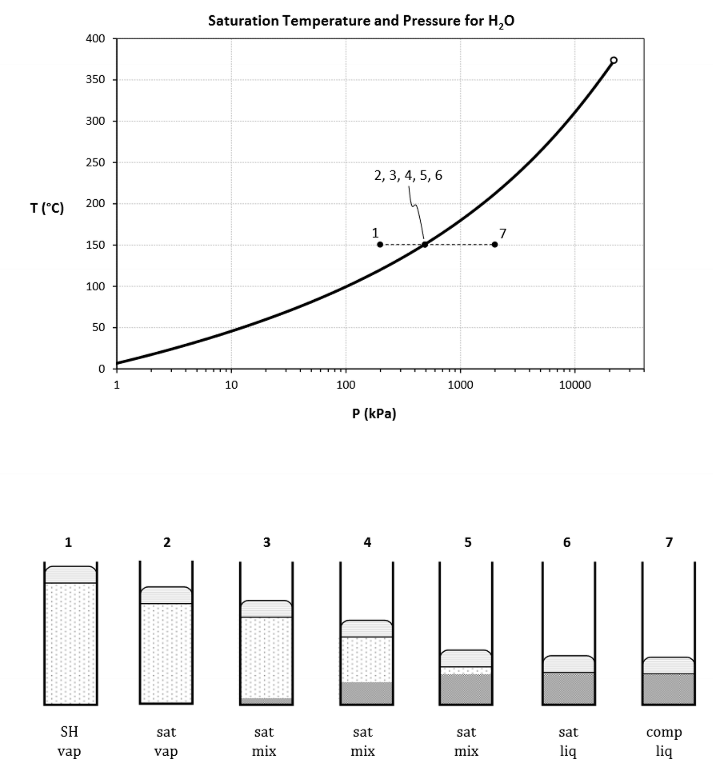
What does this diagram tell us? What even is it?
A vapour dome.
We need to get to a higher temperature to start boiling at a higher pressure.
So at higher Ps, the saturation T is higher.
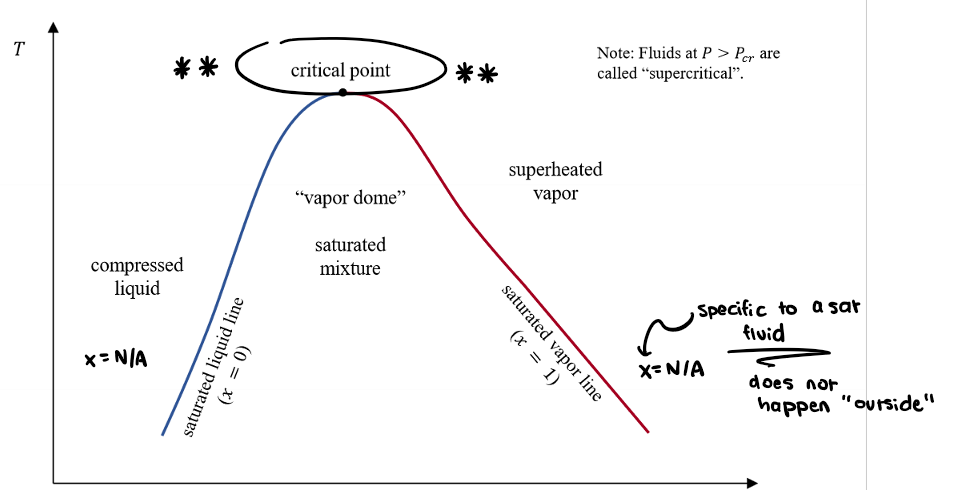
Memorize!
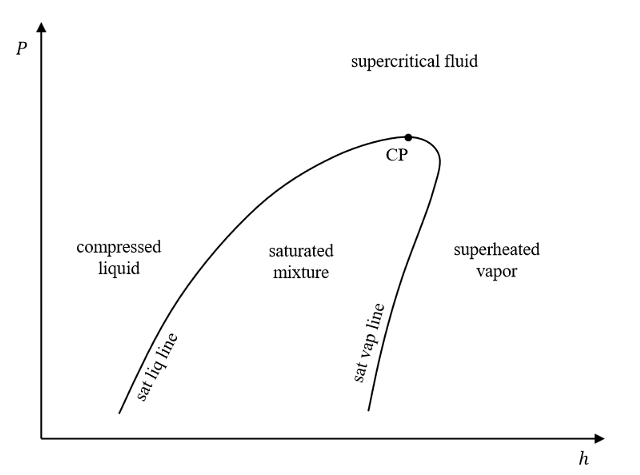
Memorize!
When fluids are in a state of P>Pcr what does this mean?
The fluids are supercritical.
What is a saturation pressure? What is a saturation temperature?
Saturation temperature is the temperature at which a fluid changes phase.
Saturation pressure is the pressure at which a fluid changes phase.
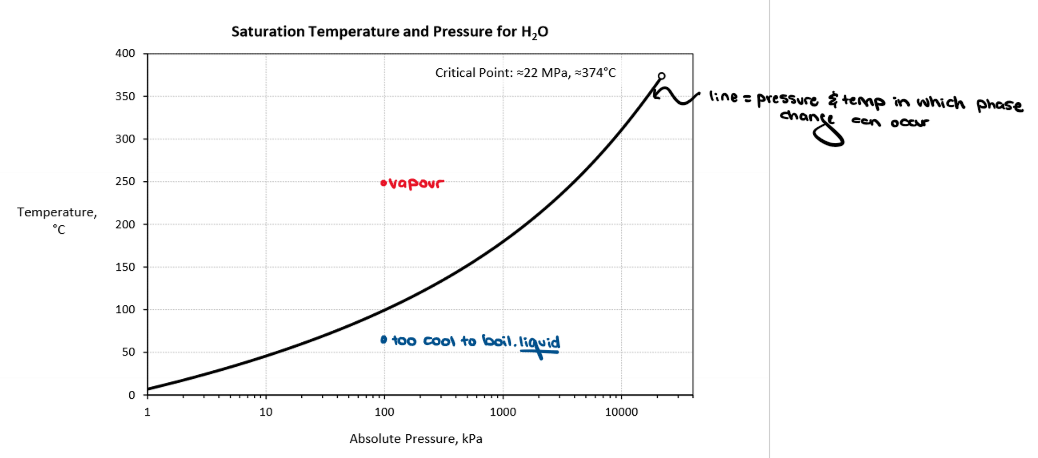
True or False. When a fluid is saturated, P and T cannot be independently varied.
True! Setting one value (P or T) sets the other
Draw a enthalpy pressure graph with isobars and isotherms.
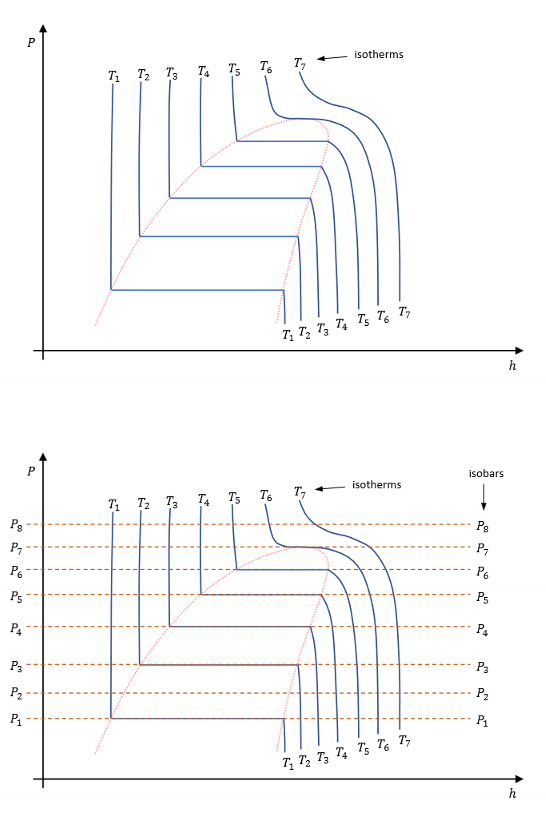
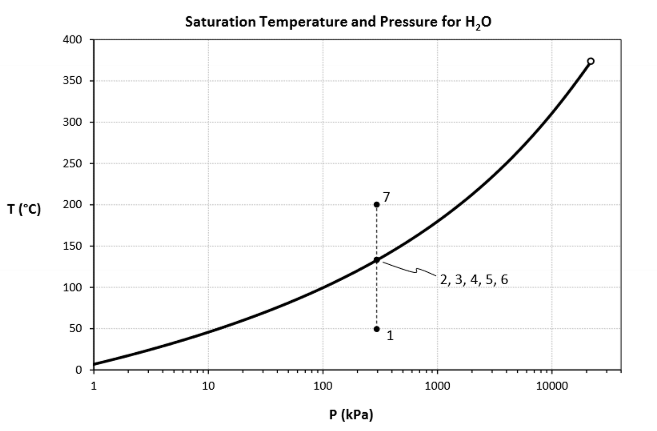
For this diagram (heating at constant pressure), for each number, draw its states
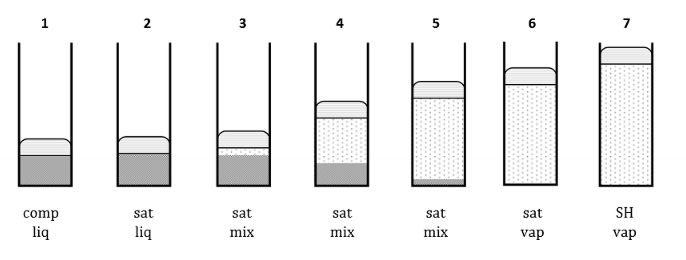
For this diagram (compression at constant pressure), for each number, draw its states
State the state postulate for a simple compressible substance.
The intensive thermodynamic state of a simple compressible substance is completely specified by two independent, intensive properties.
Check image for these properties

What is an equation of state?
A method used to relate certain thermodynamic properties of a substance while at an equilibrium condition.
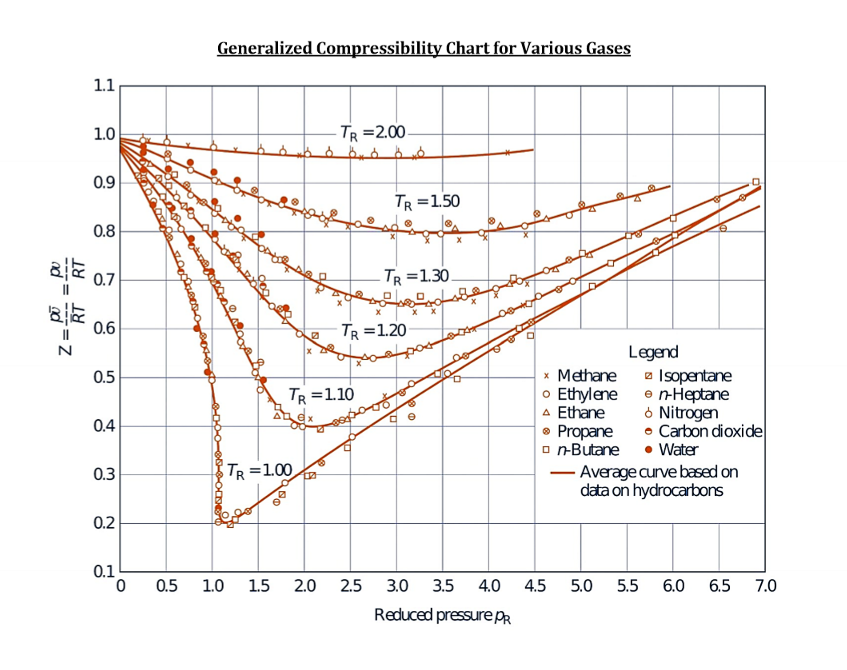
What does a compressibility factor chart look like?
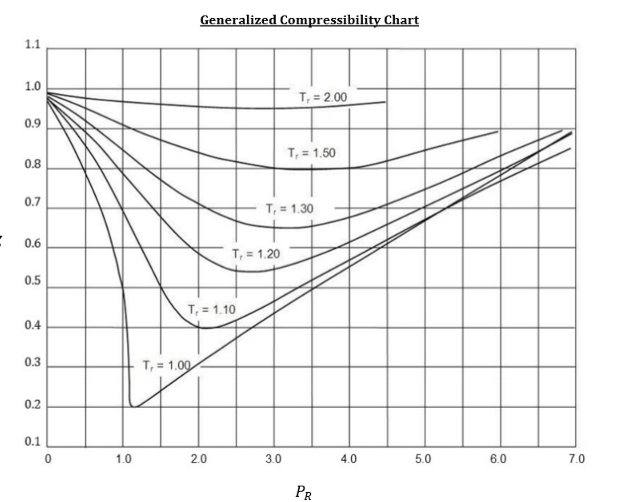
True or False. If a substance is a gas, ideal gas law can be used.
False. It can be reasonable to treat a simple compressible substance as an ideal gas if PR < 0.1 and TR ≥ 1 OR PR ≤ 7 and TR ≥ 2
Basically, when enthalpy and temperature are running parallel to each other in a dome like graphic, ideal gas can be used.
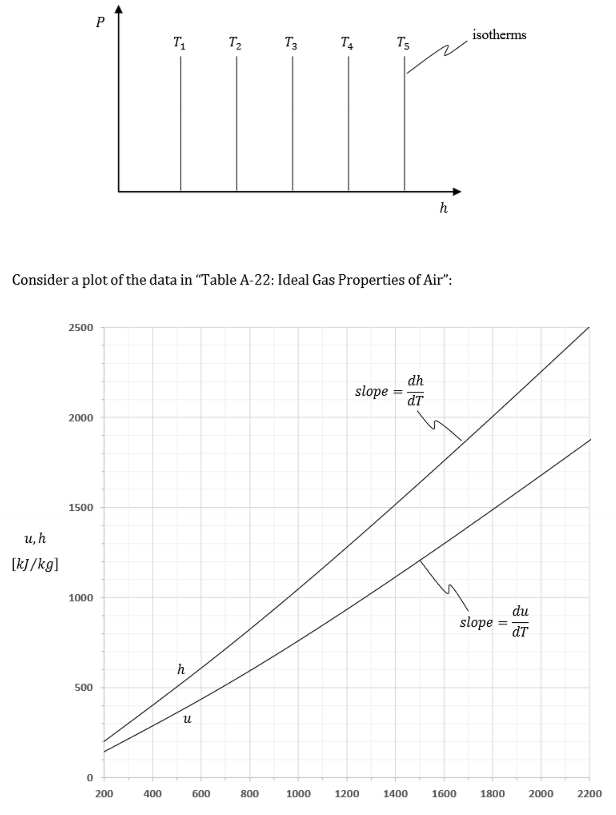
What does this graph represent?
Appearance of a region where the fluid behaves as an ideal gas
SO h = f(T)
What is an incompressible substance?
The change in specific volume of a liquid or solid not undergoing phase change is very small (where dv and dp are approx = 0). Even though there may be a significant change in pressure, this substance is still called incompressible.
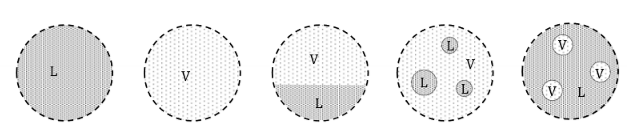
What are each of these?
sat liq
sat vapour
sat mix
sat mix
sat mix
What is a saturated liquid? What is a saturated vapour?
A saturated liquid is a liquid about to vaporize. A saturated vapour is a vapour about to condense.
A saturated fluid is a fluid about to change phase.