06 Petroleum Generation and Maturation
1/15
Earn XP
Description and Tags
Part 1 - Origin, Chemistry, Generation
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
16 Terms
Explain Inorganic origin theories.
This theory proposes that petroleum is formed through chemical reactions deep within the Earth's mantle, unrelated to biological processes.
Process:
Hydrocarbons are produced from the reaction of carbon dioxide, hydrogen, and other inorganic materials under high temperature and pressure in Earth's mantle.
These hydrocarbons migrate upward and accumulate in traps.
Explain Organic origin theories.
This theory suggests that petroleum is formed from the remains of plants, algae, and microscopic organisms (plankton) that lived millions of years ago.
Process:
Organic matter accumulates in sedimentary basins, particularly in oxygen-poor environments (to prevent decomposition).
Over time, heat, pressure, and chemical processes convert this matter into kerogen (a precursor to petroleum).
With further heat and pressure, kerogen matures into hydrocarbons (oil and gas).
Evidence for inorganic origin theories
Geographical location: most of hydrocarbon producing regions are located close to belts of tectonic activities.
Stability with depth: Corresponding to what organic theory's supporters have admitted themselves; petroleum is a fossil fuel, and there has never been a real fossil found below 16000 feet. Nowadays, there is drilling for oil reservoirs at 28000 feet or 30000 feet where there is no a fossil remains.
Evidence for organic origin theories
Presence of brine (sea water) with petroleum.
Petroleum is found only in association with sedimentary rocks. There is no petroleum associated with igneous or metamorphic rocks.
Polarized light passing through all petroleum resources undergoes a rotation that is similar to all organic oils.
Molecules in hydrocarbons are thought to be similar to that of the organic matter.
The organic carbon found in plants is depleted into C13 due to photosynthesis process. In dead organic matter, it is further depleted due to radioactive decaying. The same depletion was found in petroleum and natural gas
Sulfur
Sulfur is most abundant in the heavier crude oils and in asphalt.
It can also occur in natural gas mixtures such as the poisonous corrosive gas H2S.
Such natural gas is called sour gas, as opposed to sweet gas, where H2S is low or absent.
Sulfur is removed from crude oil and natural gas and largely used in the production of sulfate and phosphate fertilizers.
Nitrogen
Nitrogen content is generally higher in both asphalts and natural gas, when compared to crudes.
In asphalt, it occurs mostly in high molecular weight hydrocarbon compounds that are referred to as NSO compounds because they contain impurities of nitrogen, sulfur, and oxygen.
In natural gas mixtures nitrogen occurs mostly as the inactive gas N2, which lowers the heating capacity (BTU) of the natural gas.
Other compounds may also occur in natural gas mixtures, including C02 and the inert gases.
Helium (desirable)
Found in natural gas.
Helium is mainly used in cryogenics, but can also be used for pressurizing and purging gas, and as a protective atmosphere in arc welding.
Hydrocarbon Arrangement
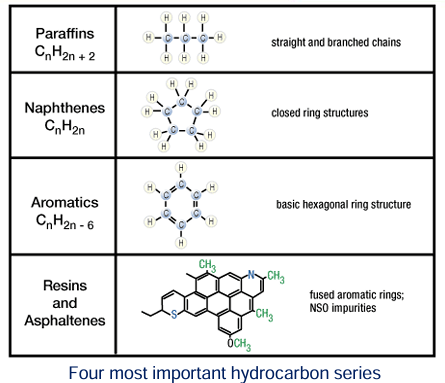
Paraffins
Paraffins occur as chain-like structures with the general formula CnH2n+2
The carbon number, "n", ranges from one in the hydrocarbon gas methane (CH4), the simplest member of the paraffin series, to over 40.
A natural gas composed of nearly pure methane is called dry gas.
Other lightweight paraffins, with carbon numbers up to 5, are also gaseous at normal temperatures and pressures.
A natural gas that contains these other heavier paraffin gases along with methane is called wet gas.
Paraffins with carbon numbers higher than 5 are normally liquid.
High molecular weight paraffins become viscous, waxy solids.
Naphthenes
Naphthenes form as closed ring structures with the basic formula CnH2n.
Compounds of th-e naphthene series have chemical and physical properties similar to equivalent paraffins with the same carbon number.
Together with the paraffins, naphthenes form the major components of most crude oils.
Aromatics
Aromatics (CnH2n-6) have a structure based on a hexagonal ring of carbons with alternate simple and double bonds.
Aromatics are responsible for the strong odor and fluorescence in oils and extracts.
Resins and Asphaltenes
Resins and asphaltenes are also composed of fused benzene-ring networks, but they contain other atoms and are not true hydrocarbons.
These impurities include the high molecular weight NSO compounds.
Resins and asphaltenes are the heaviest components of crude oil and the major components in many natural tars and asphalts.
Crude Oil Classification Triangle
Paraffinic Crude (Saturate-rich):
Positioned near the "Saturates" vertex.
Light, less dense, and easier to refine into fuels like gasoline and diesel.
Example: Brent crude.
Aromatic Crude (Aromatic-rich):
Positioned near the "Aromatics" vertex.
Contains higher amounts of aromatic hydrocarbons, useful for producing petrochemicals.
Asphaltic Crude (Resins + Asphaltenes-rich):
Positioned near the "Resins + Asphaltenes" vertex.
Heavy, viscous, and typically used for bitumen production.
Intermediate Crude:
Positioned somewhere within the triangle but not near a single vertex.
Balanced composition with varied refining and commercial potential.
Describe kerogens.
Kerogen is the fraction of sedimentary organic constituent of sedimentary rocks that is insoluble in the usual organic solvents. It's an organic substance made from ancient plants, algae, and microorganisms that got buried and changed over millions of years. Kerogen will yield different types and amounts of petroleum.
Define Maturation
A complex process through which biological molecules, created by living organisms, are converted into petroleum.
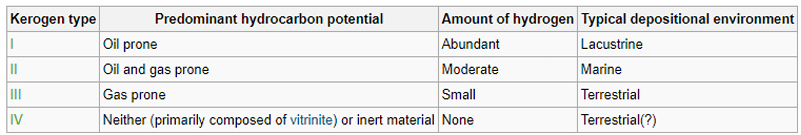
Describe 4 classes of kerogen.
Type I
• Type I kerogen is derived mostly from the remains of algae, and when it matures it yields mainly crude oil.
• It is also capable of generating the most petroleum of all the kerogen types.
Type II
• Type II kerogen consists mostly of amorphous material, derived from the bacterial and mechanical breakdown of a mixture of marine, one-celled plants and animals.
• This kerogen is also oil-prone but yields more natural gas than Type I.
• Type I and Type II Kerogens are referred to as sapropelic kerogen
Type III
• Type III kerogen, derived from the higher land plants, is sometimes known as coaly kerogen.
• The humic material in Type III kerogen has a low capacity to form oil and yields mostly natural gas.
• Type III kerogen has a low capacity to form oil and yields mostly natural gas.
Type IV
• Type IV kerogen consists mostly of inert particles that have been highly oxidized before burial, like charcoal.
• It is the rarest kerogen type and has practically no ability to generate either oil or gas.