Biochem Exam 2
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
Phosphorylation What is it and Where does it Occur?
is the process of adding a phosphate group to a molecule, often used to regulate enzyme activity and metabolic pathways in cells.
Occurs in Cytosol
Kinase
is an enzyme that catalyzes the transfer of a phosphate group from ATP to a substrate (Add Phosphate, Polar Uncharged)
Ligins
Anything that binds to a receptor
Glycosylation What is it and Where does it occur
is the enzymatic process of adding sugar moieties to proteins or lipids, which can influence cell signaling and recognition.
Occurs in golgi and ER
Post translational modification what is it and where does it occurs
post translational modifications are chemical changes made to proteins after they’re built.
via covalent bonds between the amino acid chains and added groups like (phosphate or sugar)
Common amino acids that undergo phosphorylation
S, T, Y, H
Phosphorylation What Happens?
A phosphate group is added to an amino acid by an enzyme called kinase. This can turn a protein on or off, change its shape, or alter how it interacts with other proteins.
Introduces charge to polar uncharged amino acids.
Will alter interactions with other nearby amino acids.
Glycosylation What is it?
Addition of a carbohydrate (glycan)
Glycosylation What happens?
Some proteins need glycosylation to properly fold.
Necessary for physical and chemical stability.
Can also be used for cell to cell adhesion (binding).
What happens when amino acids are modified?
When amino acids are modified, their structure changes slightly, which can affect how they work or how the protein they are part of functions. Sometimes, small chemical groups like phosphate or sugar are added to an amino acid. These changes can turn a protein on or off, make it stronger, or help it move to the right place in the cell. In simple terms, modifying amino acids changes their behavior and can change what the whole protein does in the body.
How is charge affected by PH
pH affects the charge of amino acids by changing whether their groups gain or lose hydrogen ions (H⁺). At different pH levels, amino acids can become more positive, more negative, or neutral. We can estimate the total charge by adding up the charges of all amino acids, but in reality, the actual charge can be a bit different—sometimes higher or lower—than the estimate.
pH two similar charged groups
When two groups with the same charge are close together, they repel each other, making the interaction unstable. To fix this, one group may lose a proton to reduce the repulsion. Because of this, its pKa value drops (for example, from 4.6 to 3.6), meaning it gives up the proton more easily to become more stable.
if an anion (a negatively charged ion) comes near the two positive groups, it can shield them from each other. This reduces the repulsion, meaning the pKa doesn’t change as much, and the protein structure stays more stable overall.
What happens in the opposite situation, where you have oppositely charged groups in close proximity to one another?
When oppositely charged groups are close together, they attract each other, creating a stable interaction. Because they already balance each other out, the group holding the proton is less likely to give it up. This makes its pKa go up (for example, from 4.6 to 5.6), meaning it keeps the proton more easily since the interaction is already stable.
ions can weaken that attraction, keeping the protein more balanced and less affected by pH changes.
Protein Binding
Proteins need to be able to bind other proteins and release them accordingly.
IF proteins interact too loosely, they can’t trigger the chain of events that normally happens when proteins bind.
IF proteins interact too strongly, it becomes difficult to reverse the binding.
How can proteins maintain original structure
A protein can maintain its original structure by balancing the forces between its charged groups. When repulsion or attraction between amino acids becomes too strong, nearby ions, water molecules, or other parts of the protein can help “shield” these charges. This shielding reduces stress on the protein’s shape and prevents large pKa changes. By keeping its internal charges balanced and stable, the protein is able to hold its proper 3D structure and continue working correctly.
Amino Acids that undergoes Glycosylation
Asparagine- N linked glycosylation
Serine and Theronine- O linked glycosylation
What does Phosphorylation add?
Phosphorylation adds negative charge, which can shift folding or binding
What does Glycosylation add?
Glycosylation adds bulky sugar groups which can protect or stabilize the protein
Enzymes do what?
Proteins that speed up reactions by lowering activations energy
What do Protein hormones (like insulin) do?
Signal molecules that control cell activity
Describe the protein binding of insulin
Insulin (ligand) binds to the insulin receptor (protein). This forms a protein-ligand complex, which triggers a cascade of cell signals to take up glucose
Describe DNA (Strand, Sugar, and Base Pairing)
Double stranded, stores genetic info
Sugar: Deoxyribose
Base Pairing: Adenine (A), Thymine (T), Cytosine (C), Guanine (G)
Describe RNA (Strand, Sugar, and Base Pairing)
Single stranded, helps make proteins
Sugar: Ribose
Base Pairing: Adenine (A), Uracil (U), Cytosine (C), Guanine (G)
Transcription
Translation
DNA-RNA
RNA-Protein
Bonding Hydrogen bonds and Hydrophobic bonds
Hydrogen bonds hold base pairs together
Hydrophobic interactions help stabilize the DNA helix
Inhibition Types graph

Michaelis-Menten Kinetics Graph
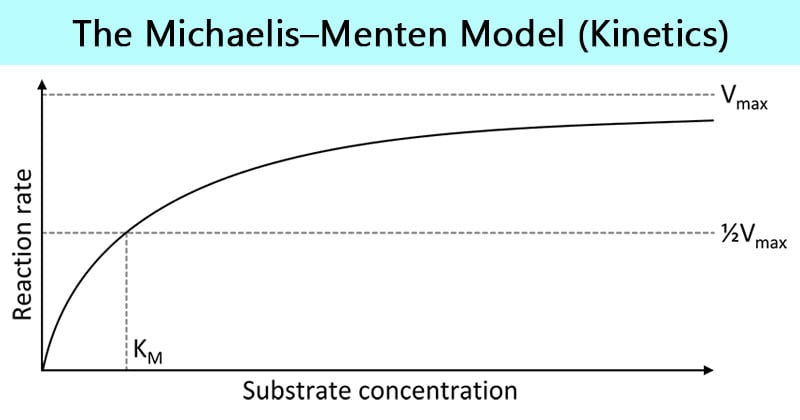
S= Substrate concentration
V0= Initial reaction rate
Vmax= Max rate (when enzyme is saturated)
Km= Substrate concentration at ½ Max (measures enzymes affinity for substrate; lower km= stronger binding)
Inhibition Types
Competitve Inhibitor: Binds active site, increases Km, Vmax unchanged
Noncompetitive: Inhibitor binds elsewhere, decreases Vmax, Km same
Draw the gel, label it, and explain why