Gen Chem - Atoms and Quanta
1/53
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
54 Terms
What was the preliminary history of the atom?
Democritus in 5th Century BCE
hypothesised that matter divided into smaller parts would eventually reach an indestructible building block (atoms)
hypothesised that atoms were indivisible and indestructible
Dalton in the 1800s
observed that chemical reactions do not create or destroy mass - chemicals react in the same ratios
theorised that chemical reactions were rearranging of atoms of different types and that compounds have fixed invariant ratios of component atoms
Both believed atoms to be indivisible
developments in the 1890s and 1930s proved subatomic structures
What is the structure of an atom?
nucleus containing protons (positively charged) and neutrons (neutral)
orbiting electrons (negatively charged)
What determines the type of element in an atom?
The number of protons in the nucleus
Determines the atomic number
How many entities are present in a mole?
6.022 × 1023 entities
Why do atoms produce different colours in flame tests?
Absorbing EM radiation causes electrons to be excited to a higher energy level
EM radiation is then emitted as photons, corresponding to a specific wavelength of light
What is the equation that links speed, wavelength and frequency for waves?
c (speed) = ʎ (wavelength) x v (frequency)
What is the equation of energy per photon?
E (energy per photon) = ɦ (Planck’s constant) x v (frequency)
What is the experimental evidence that proves wave-particle duality?
Photoelectric effect - particle
Double slit experiment - wave
How is energy quantised in a H atom?
A single photon is emitted when a H atom relaxes from an excited state to its ground state
Each state can be associated with a certain amount of energy
Difference between states must match the energy of the emitted photon
Lines on an emission spectrum correspond to transitions between energy levels - quantisation of energy
What were the principles of the Bohr atom?
Bohr put electrons in circular orbits around the nucleus
Further from the nucleus meant the electron had a higher energy
Only allowed some orbits
Each orbit has a quantum number associated with it which can only be a positive integer (1, 2, 3, 4, etc)
What are the criticisms of the Bohr atom?
Makes successful predictions for hydrogen but breaks down with multi-electron atoms
Doesn’t explain why orbits are circular or why electrons don’t relax and collapse into the nucleus
What is the purpose of the Rydberg equation?
Gives the frequency of photons emitted which correlates the frequency of photons emitted when a H atom relaxes from a higher to lower energy level
What are the parts of the Rydberg equation?
n2 - the starting energy level of the electron
n1 - the ending energy level of the electron
both n1 and n2 must be integer values
n2 must be greater than n1
RH is the Rydberg constant - 3.29 × 1015s-1
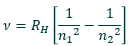
How is the absorption spectrum of H produced?
Shine a continuum of light at the sample with a mixture of wavelengths
See which wavelengths are absorbed and which pass through
End up with absorptions where emissions were recorded
Electrons undergo the same transitions in the opposite direction
What is the link between the Rydberg equation and the ionisation energy of H?
Can find the upper limit of energy levels by setting n2 to infinity in the Rydberg equation
Results in an energy of 1310kJ per mole of photons
This is the same as the ionisation energy of H
Are electrons particles or waves?
Electrons exhibit wave-particle duality so are present as both waves and particles
Can generate a stream of electrons
This stream can be described with a frequency and wavelengths
How are the wavelengths of electrons determined?
Depends on the speed electrons are projected at
Wavelengths are similar to bond lengths between atoms within molecules
Which equation links mass, speed and wavelengths of electrons?
de Broglie equation
ʎ - wavelength
ɦ - Planck’s constant
m - mass
v - frequency
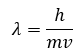
How is information about bond lengths determined?
Diffraction patterns produced when electrons of a known wavelength interact with bonds
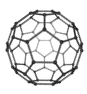
What is one of the largest molecules to exhibit wave-like behaviour?
C60 molecules (‘buckyballs’)
How are electrons as waves managed in atoms?
Electrons produce standing waves around the nucleus at specific radii
Only some wavelengths produce an ‘allowed’ standing wave
Determines the principal quantum number
What is the wavefunction of an electron?
The 3D wave of an electron
Has a value for any position relative to the nucleus of the atom
Can give positive or negative values - links to ‘phase’
Two points of a wavefunction can be ‘out of phase’ when one value is negative and the other is positive
What two items can be derived from the wavefunction of an electron?
The probability distribution function for the electron’s location
The energy of the electron
How are electron locations determined?
Fundamentally impossible to know the exact location of an electron at any given time
Can work out probabilities that it is present in a particular space
The square of the wavefunction is proportional to the probability of finding an electron at that point
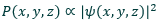
What is the probability distribution function of an electron?
Measure of the probability of an electron existing at a particular region
Equivalent to the electron density
Allows us to produce diagrams of electron probabilities in a certain space
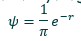
How did Schrodinger’s equations help determine electron wavelengths?
Schrodinger’s equation gives the kinetic, potential and total energy of an electron with a wavefunction
Solutions are wavefunctions with associated energies for electron total energies
Only some wavelengths and energies are possible
The value must be an integer
What does the quantum number n relate to?
The principle quantum number
Can be any whole number from 1 to infinity
The value of n directly relates to the energy level
A higher value of n means electrons have a higher energy
What does the quantum number l relate to?
Secondary quantum number
Can be any number from 0 to (n-1)
Value of l relates to a letter
Orbitals with different l values have different shapes
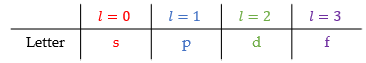
What does the quantum number ml relate to?
Magnetic quantum number
Can be any number from -l to +l
Determines the number of orbitals in a certain energy subshell
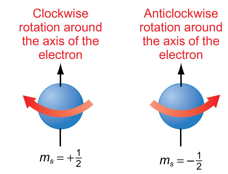
What does the quantum number ms relate to?
Fourth quantum number
Describes the orientation of electron spin
Direction of spin determines the spin magnetic quantum number of either +½ or -½
There are only two possible ms values
Depicted as up and down arrows (↑↓)
Which two ways can we identify a point in space co-ordinately?
Cartesian coordinates (x and y)
Polar/Spherical coordinates (r, θ, φ)
What can you split a full wavefunction into?
Radial wavefunction
Angular wavefunction
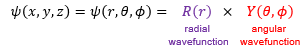
What are radial nodes?
Specific radii where the radial wavefunction has a value of 0
If the radial wavefunction = 0 the overall wavefunction must also be 0
Probability of finding an electron there will be 0
What are the two scenarios where R=0 is NOT a radial node?
non-s orbitals have R=0 at r=0
radial wavefunctions taper off to 0 as r heads to infinity
Where is the most probable distance for an electron from the nucleus?
Highest point on the radial distribution function is the most probable distance
How do radial wavefunctions vary within and between (sub)shells?
Radial wavefunctions are the same for all orbitals in a subshell (same n and l values)
Radial wavefunctions vary at differences between n and l (different shells and subshells)
Angular wavefunctions are different within subshells
How do angular wavefunctions affect the electron densities around a nucleus?
The angular wavefunction defines the shape of the wavefunction
All wavefunctions would be spherically symmetrical without angular wavefunctions
What are the numbers of angular nodes for each type of orbital?
s orbitals have 0 angular nodes - has radial nodes ‘within’
p orbitals have 1 angular node
d orbitals have 2 angular nodes
f orbitals have 3 angular nodes
the number of angular nodes = value of l
How do electron arrangements work in single electron atoms?
Electron is likely to exist in a 1s orbital as this is the lowest energy orbital
The electron absorbs energy to be excited to a higher energy level
Energy levels depend entirely on the value on n
All higher orbitals in a shell have the same energy - are degenerate
What is the Pauli Exclusion Principle?
No two electrons may have the exact same set of quantum numbers
What are the electron arrangements in a multi-electron atom?
When an atom has multiple electron the energy levels diverge for different subshells
Cannot keep putting electrons into the lowest energy orbital
Further electrons go into the lowest energy level with a vacancy
What types of forces do electrons experience in multi-electron atoms?
Attraction to the nucleus due to opposite charges
Repulsion by electrons in closer shells due to like charges
How are s orbitals most penetrating?
They have significant density close to the nucleus that is not seen for other subshells
Electrons in the s subshell have less shielding overall
Effective nuclear charge is higher for the s subshell
What is Hund’s rule?
Electrons will singly occupy orbitals of the same energy with parallel spins to maximise the total spin before any pairing occurs
What are the rules for filling orbitals?
Electrons will fill orbitals from the lowest energy level to higher levels
Up to two electrons can exist in each orbital due to electron spin and the Pauli Exclusion Principle
Electrons fill orbitals of the same subshell singly with parallel spins before pairing due to Hund’s rule
What is the effective radius of an atom determined by?
Roughly equivalent to where the outermost electrons are mostly located which is taken from radial distribution functions
What are the two main trends relating to atomic sizes?
Atoms get bigger down a group
Atoms get smaller across a period
Why do atoms get bigger down the group?
The outermost electron is in a higher energy orbital with a higher n
Higher energy orbitals mean that electron is mostly further from the nucleus so the atom is larger
Why do atoms get smaller across a period?
Due to shielding from other electrons
A proton is added to the nucleus each element across the period so nuclear charge increases by +1
Shielding from an additional electron is not enough to influence atomic size
Effective nuclear charge increases by ~+0.65 for each proton added
Electrons in the same subshell are experiencing a greater attraction from the nucleus so are attracted closer to the centre
The atoms become smaller
Why are ionisation energies always positive?
Energy is always required to remove an electron from an atom
Ionisation is an endothermic reaction
Higher value means more energy needs to be input to remove an electron
What are the two main trends relating to ionisation energies?
Increase across a period
Decrease down a group
Why do ionisation energies decrease down a group?
The outermost electrons are in a high n shell
The outermost electrons have a higher energy and are closer to being ionised
Further from the nucleus so are less tightly held by nuclear charge
Less energy needs to be input to excite the electron out of the atom
Why do ionisation energies increase across a period?
Effective nuclear charge increases so electrons are held more strongly
More energy is needed to excite the electron out of the atom
Is electron gaining a favourable or unfavourable process for an atom?
Favourable
Value of energy is negative
An exothermic reaction so energy is released when an electron is accepted